Notas / Notes
Causes of Polistes dominula (Christ, 1791) (Hymenoptera, Vespidae) colony mortality in urban areas of the south-western Iberian Peninsula
José Luis Pérez-Bote1 & Carlos Mora-Rubio2
1,2 Departamento de Anatomía, Biología Celular y Zoología, Universidad de Extremadura, Av. de Elvas s/n, 06006 Badajoz, Spain.
1 Email: jlperez@unex.es – ORCID iD: https://orcid.org/0000-0002-4042-5391
2 Email: morarubio@unex.es – ORCID iD: https://orcid.org/0000-0002-1847-3215
| |
ABSTRACT
From 2017 to 2018 we studied populations of the eusocial paper wasp Polistes dominula (Christ, 1791) in three municipalities of the south-western Iberian Peninsula, in order to identify the causes of colony mortality. The results showed that the first main cause of mortality in the study area was human activity (43.55 %). The second most important cause was abandonment of the nest (20.19 %). Only 82 of the 411 nests (19.95 %) showed signs of natural colony decline, following the emergence of reproductives.
Keywords: Polistes dominula; Hymenoptera; Vespidae; nest mortality; urban areas; Iberian Peninsula.
|
| |
RESUMEN
Causas de mortalidad de colonias de Polistes dominula (Christ, 1791) (Hymenoptera, Vespidae) en zonas urbanas del suroeste de la Península Ibérica
De 2017 a 2018 se estudiaron diversas poblaciones de la avispa eusocial Polistes dominula (Christ, 1791) en tres municipios del suroeste de la península ibérica, con la finalidad de identificar las causas de mortalidad de sus colonias. Los resultados mostraron que la causa principal de mortalidad en las zonas de estudio fue la actividad antrópica (43,55 %). La segunda causa más relevante fue el abandono del nido (20,19 %). Únicamente 82 de 411 nidos (19,95 %) demostraron un declive natural de la colonia, tras la aparición de reproductores.
Palabras clave: Polistes dominula; Hymenoptera; Vespidae; mortalidad de colonias; áreas urbanas; península ibérica.
|
In the Old World, Polistes dominula (Christ, 1791) is without doubt one of the most abundant Polistes Latreille, 1802 species. Its distribution covers the whole of central and southern Europe, although it is absent from the coldest parts. It is particularly abundant in countries around the Mediterranean basin and North African countries. Polistes dominula also occurs in Central and South Europe as far north as Latvia, but missing in Great Britain, Scandinavia, Crete and Cyprus. It has been introduced to Australia, North America and South America. The species has also been recorded from central and eastern Palaearctic regions and from India (Schmid-Egger et al., 2017). According to Castro & Carbonell Font (2016), nine species of Polistes are present in the Iberian Peninsula. Polistes dominula nests are frequent in urban areas of south-western Spain and, despite their beneficial roles as pollinators and pest controllers (Sumner et al., 2018), the presence of the wasps alarms people living near the nests. Numerous media campaigns have increased interest in this issue and have heightened the concerns of city inhabitants. This fear, which is not always fully justified, prompts people to massively informs municipal services about practically all of the observed colonies of wasps.
The aim of this study was to determine the major causes of mortality of P. dominula colonies in urban areas of the south-western Iberian Peninsula. Observations were conducted between March and October in 2017 and 2018, in three municipalities of south-western Spain (Badajoz province, Extremadura region, Spain): Olivenza (38°41′0.6″ N, 7°05′58″ W; ca. 12000 inhabitants), San Francisco de Olivenza (38°44′44″ N, 7°06′14″ W; ca. 500 inhabitants) and San Benito de la Contienda (38°37′59″ N, 7°09′26″ W; ca. 579 inhabitants). Colonies were located by actively searching the streets of the municipalities (private gardens were not sampled). Nests were observed weekly and the development or decline of each colony was recorded. Data was obtained by direct observation and using a structured questionnaire through face-to-face interviews with owners, with the aim to describe the knowledge, attitudes and practices of inhabitants towards the wasps. Direct observations were conducted from 10:00 to 13:00 h and from 17:00 to 21:00 h with the help of binoculars, so as not to disturb the behavior of the wasps or the potential predators. Direct observations were made in areas where potential predators were previously detected. The causes of colony decline were divided into five categories: (1) human activities, (2) nest abandonment by foundress, (3) natural causes (natural colony decline after the emergence of fertile females and males), (4) predation, and (5) unknown causes. A nest was considered abandoned when its construction was interrupted during the first phases of the colony cycle. In these cases, nests appear with a very low number of cells. Data on nests destroyed by humans were obtained through owner surveys. Where possible, information was also obtained from workers working on the streets (builders, electricians, painters). Predation was quantified in two ways: direct observation (ants, vertebrates) and observation of damage to nests or by detection of remains below nests (see Kozyra & Baraniak, 2016). In this study we only consider rain as the only natural cause of nest disappearance. In the unknown category are included those nests to which we could not attribute to any of the causes listed above to justify their disappearance. These causes can be some of those previously mentioned or others, such as parasites, diseases, lack of food or threats from predators.
The number of nests destroyed by humans was 179 (43.55 %). The nests were destroyed by physical knock-down (72.62 %) much more often than with insecticides, fire (or smoke) or by plugging up the nest (when they are located in cracks) probably due to the fact that, in most cases, the nests are located at 3-4 m above the ground. In Piscataway (New Jersey, USA), Fowler (1983) found that 45 % of the nests of Polistes studied were destroyed by man (of which 64.3 % were destroyed by treating with pesticides and the remaining 35.7% by knock down). Reed & Vinson (1979) examined the direct effects of human intervention on colony survivorship in Texas, finding up to 50 % of the nests were destroyed by humans.
Of the 411 nests observed, 83 (20.19 %) were abandoned by their foundress (Fig. 1). The abandonment rate was highest in the first 2-3 weeks (54.21 %) of the colony cycle and slowed down with the appearance of the first workers, as previously pointed out by Tibbetts & Reeve (2003). Several factors have been mentioned to explain the abandonment of nests by the foundress of Polistes species: predation (Judd, 1998; Kozyra & Baraniak, 2016), parasitism (Cervo & Turillazzi, 1985), scarcity of available food (Nonacs & Reeve, 1995) and adverse weather conditions (Miyano, 1980). In addition, Tibbetts & Reeve (2003) found that smaller singly founded nests failed more than larger singly founded nests, thus indicating that the cause of foundress disappearance may be more complicated than foundress death. These authors pointed out that perhaps foundresses on small less-successful nests abandon these nests to pursue other reproductive strategies. In this way, Nonacs & Reeve (1995) found that solitary foundresses readily pursue alternative strategies such as usurping, adopting or joining other nests.
|
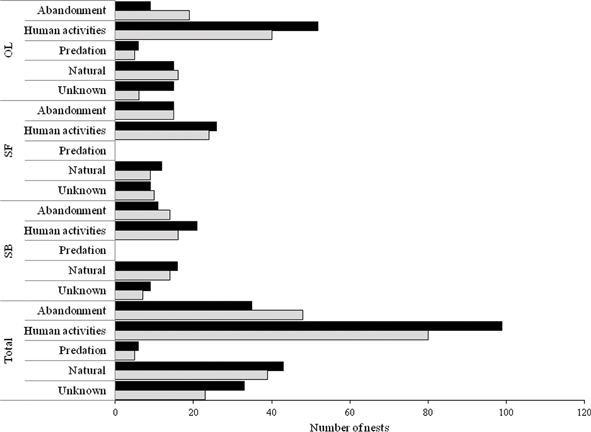 Fig. 1.— Types and numbers of Polistes dominula (Christ, 1791) nest losses in 2017 (black bars) and 2018 (grey bars) (OL: Olivenza, SF: San Francisco de Olivenza, SB: San Benito de la Contienda). Fig. 1.— Types and numbers of Polistes dominula (Christ, 1791) nest losses in 2017 (black bars) and 2018 (grey bars) (OL: Olivenza, SF: San Francisco de Olivenza, SB: San Benito de la Contienda).
Fig. 1.— Causa y número de nidos de Polistes dominula (Christ, 1791) desaparecidos en 2017 (barras negras) y 2018 (barras grises) (OL: Olivenza, SF: San Francisco de Olivenza, SB: San Benito de la Contienda).
|
|
Natural causes accounted for 19.95 % of total mortality of P. dominula colonies. Kozyra & Baraniak (2016) found that only 11 of the 308 nests studied (3.6 %) near Poznan (Poland) showed signs of natural colony decline. This corroborates the fact that in urban environments the nests are more protected from adversity than in the countryside.
Ant predation was detected in two nests. Miyano (1980) pointed out that ants are a serious threat to P. chinensis antennalis Pérez, 1905 in Japan, especially during the early phases of the nesting period. Rusina (2011) and Kozyra & Baraniak (2016) also reported cases of ant predation on field colonies of P. nimpha (Christ, 1791) and P. gallicus Linnaeus, 1761, respectively. Direct attacks by the Moorish Gecko (Tarentola mauritanica Linnaeus, 1758) were observed in three cases. This reptile is very common in anthropogenic habitats of the Iberian Peninsula. They are mostly insectivorous and forage widely or adopt an ambush strategy to obtain prey (Hódar et al., 2006). We suspected that at least six nests were predated by insect-eating birds. Specifically, by the Common House Martin (Delichon urbicum Linnaeus, 1758), an insectivorous migrating bird that build its nests in roofs and other human artifacts. On several occasions we saw Common House Martins feeding near P. dominula nests. In Italy, Cervo & Turillazzi (1985) pointed out that the failure of field colonies of P. nimpha was mainly due to bird predation (60.5 % of the studied cases).
The causes of mortality of 56 nests were unknown. We suspect that the unknown causes of mortality were mainly climatic conditions (e.g., the extreme temperatures in the summer of 2017 and the storms and heavy rains in the spring of 2018), but we cannot reject the factors previously mentioned. Miyano (1980) could not explain the failure in a high percentage of P. chinenis antennalis nests and points out that they probably failed because they lost their queens. In Poland, the unknown cause of mortality of P. nimpha (Christ, 1791) colonies oscillated between 20.4 and 39.4 % (Kozyra & Baraniak, 2016).
In terrestrial ecosystems, hymenopterans are one of the most seriously threatened insect groups (Sánchez-Bayo & Wyckhuys, 2019). The main drivers of species declines appear to be in order of importance: 1) habitat loss and conversion to intensive agriculture and urbanisation; 2) pollution, mainly that by synthetic pesticides and fertilizers; 3) biological factors, including pathogens and introduced species; and 4) climate change. However, in urban habitats man´s direct intervention seems to be a key factor in the decline of hymenopterans, circumstance that may be corrected by educational programs.
AcknowledgementsTOP
We sincerely thank the people of Olivenza, San Francisco de Olivenza and San Benito de la Contienda for help and collaboration during the fieldwork of this study.
ReferencesTOP
| ○ |
Castro, L. & Carbonell Font, R., 2016. Presencia de Polistes bischoffi (Hymenoptera, Vespidae) en Cataluña (Península Ibérica). Butlletí de la Institució Catalana d’Història Natural, 80: 25–27. |
| ○ |
Cervo, R. & Turillazzi, S., 1985. Associative foundation and nesting sites in Polistes nimpha. Naturwissenschaften, 72(1): 48–49. https://doi.org/10.1007/BF00405334 |
| ○ |
Fowler, H.G., 1983. Human effects on nest survivorship of urban synanthropic wasps. Urban Ecology, 7: 137–143. https://doi.org/10.1016/0304-4009(83)90032-3 |
| ○ |
Hódar, J., Pleguezuelos, J. M., Villafranca, C. & Férnandez-Cardenate, J. R., 2006. Foraging mode of the moorish gecko Tarentola mauritanica in an arid environment: inferences from abiotic setting, prey availability and dietary composition. Journal of Arid Environments, 65: 83–93. https://doi.org/10.1016/j.jaridenv.2005.08.006 |
| ○ |
Judd, T. M., 1998. Defensive behavior of colonies of the paper wasps, Polistes fuscatus, against vertebrate predators over the colony cycle. Insectes Sociaux, 45: 197–208. https://doi.org/10.1007/s000400050080 |
| ○ |
Kozyra, K. B. & Baraniak, E., 2016. Causes of mortality of Polistes nimpha colonies. Insectes Sociaux, 63: 481–482. https://doi.org/10.1007/s00040-016-0484-0 |
| ○ |
Miyano, S., 1980. Life tables of colonies and workers in a paper wasp, Polistes chinensis antennalis, in Central Japan (Hymenoptera: Vespidae). Researches on Population Ecology, 22: 69–88. https://doi.org/10.1007/BF02513536 |
| ○ |
Nonacs, P. & Reeve, H. K., 1995. The ecology of cooperation in wasps: causes and consequences of alternative reproductive decisions. Ecology, 76: 953–967. https://doi.org/10.2307/1939359 |
| ○ |
Reed, H. C. & Vinson, S. B., 1979. Nesting ecology of paper wasps (Polistes) in a Texas urban area (Hymenoptera: Vespidae). Journal of the Kansas Entomological Society, 52: 673–689. |
| ○ |
Rusina, L. Yu., 2011. Some aspects of interrelations between ants (Hymenoptera, Formicidae) and polistine wasps (Hymenoptera, Vespidae). Entomological Review, 91: 241–252. https://doi.org/10.1134/S0013873811020126 |
| ○ |
Sánchez-Bayo, F. & Wyckhuys, K. A. G., 2019. Worldwide decline of the entomofauna: A review of its drivers. Biological Conservation, 232: 8–27. https://doi.org/10.1016/j.biocon.2019.01.020 |
| ○ |
Schmid-Egger, C., van Achterberg, K., Neumeyer, R., Morinière, J. & Schmidt, S., 2017. Revision of the West Palaearctic Polistes Latreille, with the descriptions of two species – an integrative approach using morphology and DNA barcodes (Hymenoptera, Vespidae). ZooKeys, 713: 53–112. https://doi.org/10.3897/zookeys.713.11335 |
| ○ |
Sumner, S., Law, G. & Cini, A., 2018. Why love bees and hate wasps. Ecological Entomology, 43: 836–845. https://doi.org/10.1111/een.12676 |
| ○ |
Tibbetts, E. A. & Reeve, H. K., 2003. Benefits of foundress associations in the paper wasp Polistes dominulus: increased productivity and survival, but no assurance of fitness returns. Behavioral Ecology, 14(4): 510–514. https://doi.org/10.1093/beheco/arg037 |
Fig. 1.— Types and numbers of Polistes dominula (Christ, 1791) nest losses in 2017 (black bars) and 2018 (grey bars) (OL: Olivenza, SF: San Francisco de Olivenza, SB: San Benito de la Contienda).