Notas / Notes
Unusual habitat for Bathynellacea (Crustacea, Malacostraca): first record of this groundwater crustacean in the mesovoid shallow substratum (MSS)
Ana I. Camacho1,* & Vicente M. Ortuño2
1Museo Nacional de Ciencias Naturales (CSIC). Dpto. de Biodiversidad y Biología Evolutiva. C/ José Gutiérrez Abascal 2. 28006 Madrid (Spain). ORCID iD: https://orcid.org/0000-0003-0596-7678
2Grupo de Investigación de Biología del Suelo y Ecosistemas Subterráneos. Dpto. de Ciencias de la Vida, Facultad de Ciencias, Universidad de Alcalá. Alcalá de Henares, Madrid (Spain); vicente.ortuno@uah.es; ORCID iD: https://orcid.org/0000-0001-5734-1621
* Corresponding author: mcnac22@mncn.csic.es
| |
ABSTRACT
First world record of a crustacean (Malacostraca, Bathynellacea) that lives exclusively in groundwater in an unusual habitat, the mesovoid shallow substratum (MSS). The MSS is a terrestrial subterranean medium with high and constant relative humidity. Specimens of the family Parabathynellidae have been found in sampling devices set to collect terrestrial subterranean fauna in the MSS of Sierra de Guadarrama National Park (Madrid, Spain). Two species belonging to two different genera, Hexabathynella nicoleiana Camacho, 1986 and Hexaiberobathynella mateusi (Galhano, 1967), already known to occur in the province of Madrid, have been identified by morphological study, whereas their
18S gene sequences confirmed their generic ascription.
Keywords: MSS; groundwater fauna; Parabathynellidae; Sierra de Guadarrama; Spain.
|
| |
RESUMEN
Hábitat inusual para Bathynellacea (Crustacea, Malacostraca): primer registro de este crustáceo de agua subterránea en el sustrato superficial mesovoide (MSS)
En este trabajo se documenta por primera vez el hallazgo de ejemplares de un crustáceo (Malacostraca, Bathynellacea) que vive exclusivamente en las aguas subterráneas de todo el mundo, en un hábitat inusual: el medio subterráneo superficial (MSS), un medio terrestre sin luz y saturado de humedad. Especímenes de la familia Parabathynellidae han sido encontrados en dispositivos de muestreo dispuestos para la recogida de fauna subterránea terrestre en el MSS del Parque Nacional de la Sierra de Guadarrama (Madrid, España). Se han identificado dos especies, de dos géneros diferentes, Hexabathynella nicoleiana Camacho, 1986 y Hexaiberobathynella mateusi (Galhano, 1967), mediante estudio morfológico. Las secuencias del gen 18S de varios ejemplares confirman su adscripción genérica. Estas especies eran ya conocidas en la provincia de Madrid.
Palabras clave: MSS; fauna acuática subterránea; Parabathynellidae; Sierra de Guadarrama; España.
|
The mesovoid shallow substratum (“milieu souterrain superficiel”; MSS or terrestial SSHs) (Juberthie et al., 1980, 1981; Uéno, 1980, 1981; Pipan & Culver, 2012) is a terrestrial habitat that consist of a network of voids and interstices located above the deep subterranean domain and immediately below the soil, lightless and highly humid (Mammola et al., 2016). It includes talus and scree slopes in both carbonate (soluble) and non-carbonate rocks, including volcanic rocks. Also are an aquatic SSHs that include epikarst and the hypotelminoheic realm (Pipan & Culver, 2012). The epikarst, uppermost layer of karst, may be air or water filled and occupies a similar vertical position to that of the MSS. The perched aquifers (isolated wetlands) (hypotelminorheic by Mestrov, 1962) are the most superficial of SSHs and together with epikarst and MSS expand the scope of subterranean habitats (Pipan & Culver, 2012). The characteristics of these environments are very different as different are the faunas that can inhabit them (Pipan & Culver, 2012). The common characteristics are absence of light, high relative humidity and attenuated fluctuations in temperature throughout the year. In the MSS there is a rich and diverse terrestrial fauna composed of both hypogean species with different degrees of adaptation to the subterranean environment as well as epigean and endogean species that transit between the surface and the subterranean environment (Pipan & Culver, 2012; Ortuño et al., 2013; Mammola et al., 2016). The MSS (terrestrial SSHs) has been hardly studied, mostly in the French Pyrenees and other (noncalcareous) areas from Europe, Japan and China (Gers, 1992; Juberthie & Decu, 1994; Ruzicka et al., 1995). Medina & Oromí (1990) extended the habitat to include volcanic terrain in the Canary Islands (López & Oromí, 2010; Pipan et al., 2011).
Here, we report for the first time the presence of Bathynellacean crustaceans in the MSS. These aquatic animals live exclusively in groundwater (stygobionts). To date they have been found in caves (gours, puddles, ponds, lakes, rivers, etc.), seepage springs, sources, artificial wells, aquifers (mine bores, bore holes, irrigation waters) and in the hyporheic habitat associated to epi- and hypogean rivers. Never before had they been found in a terrestrial environment like the MSS as defined.
Bathynellacea were collected with traps set to sample terrestrial subterranean fauna in the Sierra de Guadarrama National Park (Madrid, Spain) (Fig. 1). The study area is located in the Central System mountain range of the Iberian Peninsula, which peaks from 1200 to 2428 m above sea level (a.s.l). Orthogneiss of metamorphic quartz-feldspathic origin are the geological substratum predominant in the Park (Vialet et al., 1987), and appears as colluvial and moraine deposits whose subsoil has been sampled as described elsewhere (Baquero et al., 2017; Ledesma et al., 2019; Ortuño et al., 2019). Thirty-three subterranean sampling devices (SSD) set in 33 scree slopes covered most of the Park area (Fig. 1). Each SSD consisted of a PVC tube 1 m long and 11 cm in diameter with perforations of 8 mm in diameter arranged along 40 cm from midway of the cylinder to its base. The cylinders were inserted vertically into a previously excavated hole. A pitfall trap baited with very smelly cheese and filled with 1,2-propanediol was deployed within each cylinder and the whole set covered as shown (see Fig. 2A-B). The sampling period, as a whole, covered from May 20, 2015, to October 14, 2016, but the SSD-32 (Fig. 2C) worked from July 9, 2015 to October 28, 2016 (Fig. 3). In this SSD, 12 specimens of the family Parabathynellidae were collected (7 ♂♂, 4 ♀♀ and a juvenile specimen displaying only 4 pairs of thoracopods) (Fig. 4A) during the first sampling period (09/07/2015 to 22/10/2015) (Fig. 3). They were preserved in 90% ethanol to carry out morphological and molecular studies.
|
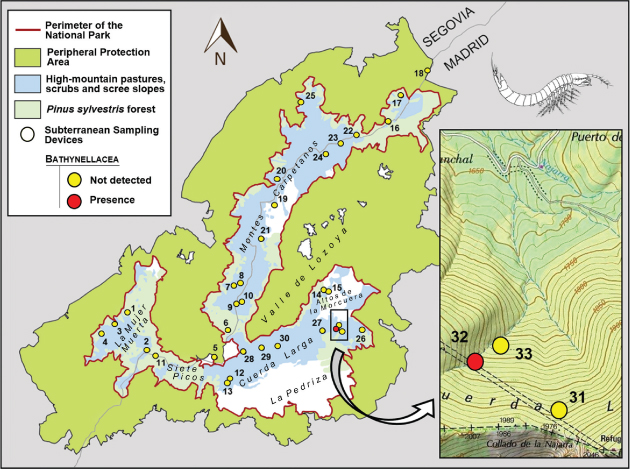 Fig. 1.— Distribution of subterranean sampling devices in the Sierra de Guadarrama National Park (Madrid, Spain) and (in red Bathynellacea specimens found). Fig. 1.— Distribution of subterranean sampling devices in the Sierra de Guadarrama National Park (Madrid, Spain) and (in red Bathynellacea specimens found).
Fig. 1.— Mapa del Parque Nacional de la Sierra de Guadarrama, Madrid, España y distribución de los dispositivos de muestreo del MSS (en rojo las trampas donde se han encontrado ejemplares de Bathynellacea).
|
|
|
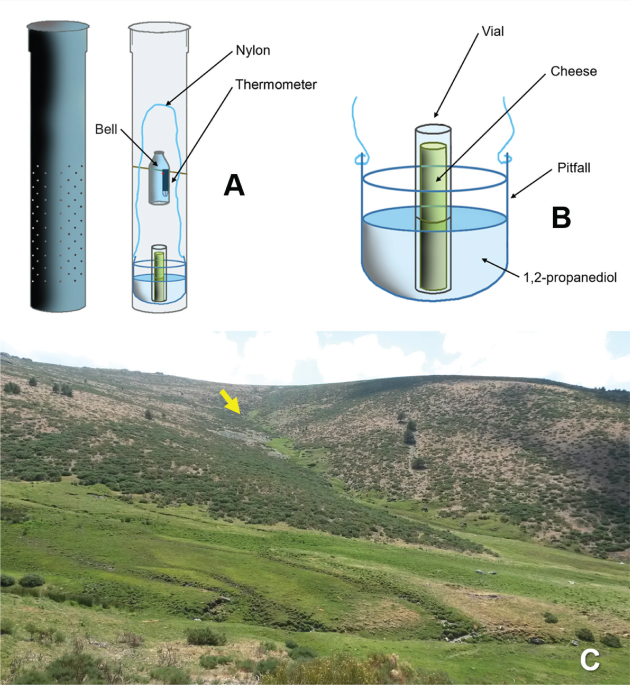 Fig. 2.— A) Sampling devices. B) Pitfall trap. C) Location of SSD-32 trap were the Bathynellacea specimens have been found. Fig. 2.— A) Sampling devices. B) Pitfall trap. C) Location of SSD-32 trap were the Bathynellacea specimens have been found.
Fig. 2.— A) Dispositivo de muestreo. B) Esquema de la trampa usada. C) Área donde han sido encontrados los especímenes de Bathynellacea en la trampa SSD-32.
|
|
|
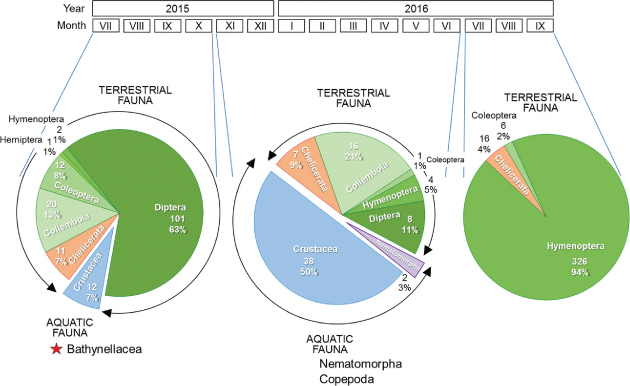 Fig. 3.— Outline of samples taken and % of fauna found at each period. Fig. 3.— Outline of samples taken and % of fauna found at each period.
Fig. 3.— Esquema de muestreos realizados y % de fauna encontrados en cada periodo.
|
|
|
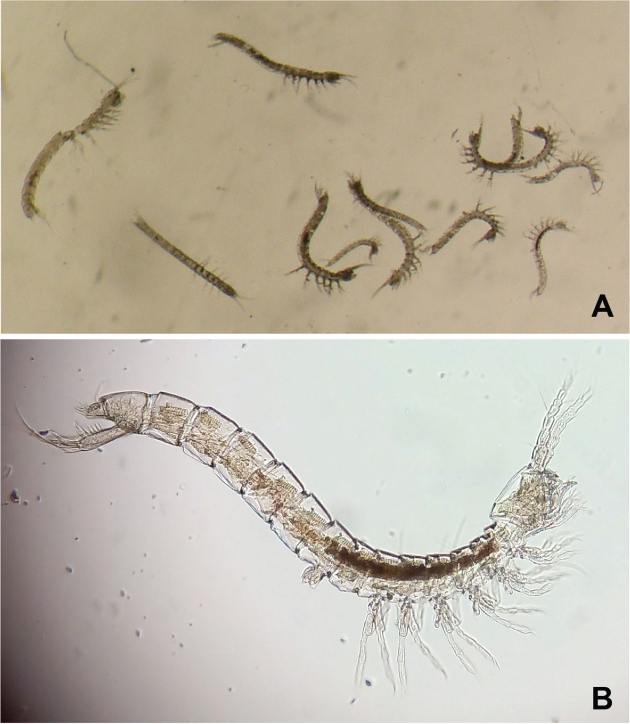 Fig. 4.— A) 12 specimens of Bathynellacea (7 ♂♂, 4 ♀♀ and a juvenile specimen) found in SSD-32 trap; B) Hexaiberobathynella mateusi, ♂. Fig. 4.— A) 12 specimens of Bathynellacea (7 ♂♂, 4 ♀♀ and a juvenile specimen) found in SSD-32 trap; B) Hexaiberobathynella mateusi, ♂.
Fig. 4.— A) Los 12 ejemplares de Bathynellacea (7 ♂♂, 4 ♀♀ y un juvenil) encontrados en la trampa SSD-32; B) Hexaiberobathynella mateusi, ♂.
|
|
We selected three whole specimens and the abdomen of another five to extract DNA (see Table 1). We succeed to extract DNA from six animals, and 18S rRNA gene sequences from three of them. We failed to sequence the COI gene in all the extracts. DNA extraction and amplification methods appear described in Camacho et al. (2018). The extracted DNA was deposited in the Tissues and DNA Collection of the MNCN (voucher numbers of the specimens shown in Table 1).
Table 1.— Specimens studied, voucher number of MNCN Collections (AIC voucher, author collections) and result of 18S sequenced.
Tabla 1.— Especímenes estudiados con los números correspondientes de la colección del autor, AIC y de las Colecciones de Artrópodos y de Tejidos y ADN del Museo Nacional de Ciencias Naturales (MNCN) de Madrid (CSIC) y resultados de la secuenciación del gen 18S.
| |
Sex |
Voucher AIC Slide-DNA |
Voucher MNCN/ARTP |
Voucher MNCN/DNA |
Gen 18S |
| Hexabathynella nicoleiana |
juvenile |
------- 1040 |
— |
54698 (whole) |
yes |
| |
♂ |
------- 1044 |
— |
54702 (whole) |
yes |
| |
♂ |
2688-1045 |
20.04/20128 |
54703 (abdomen) |
no |
| Hexaiberobathynella mateusi |
♂ |
2686-1041 |
20.04/20129 |
54699 (abdomen) |
yes |
| |
♂ |
2686-1041 |
20.04/20129 |
54699 (abdomen) |
yes |
| |
♂ |
------- 1043 |
— |
54701 (whole) |
no |
| |
♂ |
2689- ------- |
20.04/20131 |
— |
no |
| |
♂ |
2690- ------ |
20.04/20132 |
— |
no |
| |
♀ |
2691- ------- |
20.04/20133 |
— |
no |
| |
♀ |
2692- ------- |
20.04/20134 |
— |
no |
| |
♀ |
2702-1055 |
20.04/20135 |
54713 (abdomen) |
no |
| |
♀ |
2703-1056 |
20.04/20136 |
54713 (abdomen) |
no |
For the morphological study, nine specimens were completely dissected (Table 1) and the appendages preserved as permanent slides (special metal slides, glycerine-gelatine stained with methylene blue and paraffin as mounting medium; see Perina & Camacho, 2016). The morphological examination was performed using an oil immersion lens (at 1000x magnification) with a Zeiss interference contrast microscope equipped with a drawing tube. Photographs were taken with a Leica camera (LEICA MC170 HD) attached to the microscope with 400x magnification and 1000x. The specimens prepared on permanent slides are deposited in the Collection of Arthropoda of the Museo Nacional de Ciencias Naturales-ARTP/MNCN-, Madrid, Spain (see voucher in Table 1).
We identified two species of the Parabathynellidae family in the collected material: Hexaiberobathynella mateusi (Galhano, 1967) (9 specimens; 5 ♂♂ and 4 ♀♀) and Hexabathynella nicoleiana Camacho, 1986 (3 specimens; 2 ♂♂ and 1 juvenile) (Fig. 4B; Table 1).
Morphologically both genera are very different, but the differences are only observed in the dissected specimens and not when they are observed under the stereo-microscope (Fig. 4A). The species belong to the only two genera characterized by the display of only six pairs of thoracopods. The rest of known genera (85 currently; Camacho, 2019) display seven pairs of thoracopods. Hexaiberobathynella Camacho & Serban, 1998 display a 7-segmented antennule (AI) and a 3-segmented antenna (AII) (Fig. 4B); while Hexabathynella Schminke, 1972 has a 6-segmented AI and a 5-segmented AII, in addition to many other relevant differences (Table 2).
Table 2.— Differences and similarities between the two species studied: Hexabathynella nicoleiana Camacho, 1986 and Hexaiberobathynella mateusi (Galhano, 1967).
Tabla 2.— Semejanzas y diferencias entre las dos especies estudiadas: Hexabathynella nicoleiana Camacho, 1986 y Hexaiberobathynella mateusi (Galhano, 1967)
| |
H. nicoleiana |
Hi. mateusi |
| Antennule: number of segments |
6 |
7 |
| Antennal organ |
Present |
Absent |
| Antenna: number of segments |
5 |
3 |
| Labrum: number of teeth |
10 |
8 |
| Md: teeth pars incisiva |
4-5 |
4-6 |
| teeth pars molaris |
5 |
7-8 |
| distal spine modified |
Y |
N |
| Mx.I: teeth distal endite |
4 |
6 |
| teeth proximal endite |
3 |
4 |
| Mx.II: setae segment 1 |
2 |
0 |
| setae segment 2 |
4 |
4 |
| setae segment 3 |
13 |
14 |
| Male Th. VIII: shape |
Elongated |
Almost square |
| Exopod |
Long |
Small |
| Female Th. VIII: size |
Medium |
Small |
| Spines |
0 |
1 |
| Uropod: sympod |
5+1 spines |
5-7+1spines |
| setae exopod |
3 barbed |
4 barbed |
| setae endopod |
1+1 plumose |
2 barbed |
| Furcal rami |
3 spines |
5-8 spines |
| Pleotelson: setae |
1 |
1 |
| Anal operculum |
Large |
Medium size |
The molecular results have confirmed the morphological identification of both genera. We have succeeded in sequencing the
18S gene in extracts corresponding to three specimens (see Table 1). Comparison with sequences we have in our database (Hexabathynella sevillaensis Camacho, 2005, Hexaiberobathynella hortezuelensis Camacho & Serban, 1998 and Hi. mateusi) has shown that the new sequences correspond, without any doubt, to these two genera.
The confirmation of the identification to species of the studied material was not possible based on gene sequences since we do not have sequences of specimens from the type localities of both taxa: Douro River mouth (for Hi. mateusi) and Jarama River (for H. nicoleiana; Table 3). Furthermore, we failed to get COI sequences of the two species studied. Since cryptic species are frequent among Bathynellacea
(Camacho et al., 2011) we cannot discard that although we have morphologically identified the specimens as belonging to these two species, it could be that they are sister species. What is unquestionable is the generic ascription of both species.
Table 3.— Populations of Hexabathynella Schminke, 1972 and Hexaiberobathynella Camacho & Serban, 1998 in the Iberian Peninsula (updated data of Camacho & Serban, 2000; Camacho, 2003, 2006, 2019; Camacho et al., 2013a, b, 2014, 2017 and new samplings). * Type locality; **Species confirmation by DNA analysis. Number in parentheses as figure 5.
Tabla 3.— Poblaciones de las especies de los géneros Hexabathynella Schminke, 1972 y Hexaiberobathynella Camacho & Serban, 1998 encontrados en la Península Ibérica (datos actualizados de Camacho & Serban, 2000; Camacho, 2003, 2006, 2019; Camacho et al., 2013a, b, 2014, 2017 y de nuevos muestreos). * Localidad tipo de cada una de las especies; **Especie confirmada mediante análisis de ADN. Los números entre paréntesis corresponden a los del mapa de la figura 5.
| Species |
Habitat |
Locality |
Town |
Province |
Country |
| Hexabathynella |
|
|
|
|
|
| H. minuta (1) |
Interstitial |
*Duero River |
Zebreiros |
|
Portugal |
| (2) |
Interstitial |
Rivera de Huelva Stream |
Embalse de la Minilla |
Sevilla |
Spain |
| (3) |
Interstitial |
Pinhao Stream |
Balsa |
|
Portugal |
| H. nicoleiana (4) |
Interstitial |
*Jarama River |
Torrelaguna |
Madrid |
Spain |
| (5) |
Interstitial |
Jarama River |
Talamanca del Jarama |
Madrid |
Spain |
| (6) |
Interstitial |
Jarama River |
Pontón de la Oliva |
Madrid |
Spain |
| (7) |
Interstitial |
**Tajuña Stream |
Orusco |
Madrid |
Spain |
| (8) |
MSS |
**Ptº Morcuera |
Sierra de Guadarrama |
Madrid |
Spain |
| H. sevillaensis (9) |
Cave |
* **Santiago el Grande |
Constantina |
Sevilla |
Spain |
| H. valdecasasi (10) |
Interstitial |
Torcón Stream |
San Martín de Montalbán |
Toledo |
Spain |
| Hexabathynella sp (11) |
Interstitial |
Astillas Stream |
Gredos |
ávila |
Spain |
| Hexaiberobathynella |
|
|
|
|
|
| Hi. mateusi (12)
|
Interstitial |
*Duero River |
Near Porto |
|
Portugal |
| (13) |
Interstitial |
Mondego River |
Coimbra |
|
Portugal |
| (14) |
Interstitial |
Cavado River |
Barcelos |
|
Portugal |
| (15) |
MSS |
**Ptº Morcuera |
Sierra de Guadarrama |
Madrid |
Spain
|
| (16) |
Cave |
Reguerillo |
Patones |
Madrid |
Spain |
| (17) |
Interstitial |
Picnic Area |
Talamanca del Jarama |
Madrid |
Spain |
| (18) |
Interstitial |
Bridge |
Torrelaguna |
Madrid |
Spain |
| (19) |
Interstitial |
Pusa Stream |
Santa Ana de Pusa |
Toledo |
Spain |
| (20) |
Interstitial |
Valdehornos Stream |
Montes de Toledo |
Toledo |
Spain |
| (21) |
Interstitial |
Tajo River |
Peñalen |
Guadalajara |
Spain |
| (22) |
Interstitial |
Tajo River |
Zaorejas |
Guadalajara |
Spain |
| (23) |
Interstitial |
Hoz Seca Stream |
Peralejo de las Truchas |
Guadalajara |
Spain |
| (24) |
Interstitial |
Sorbe Stream |
---------- |
Guadalajara |
Spain |
| (25) |
Interstitial |
Ucero Stream |
Ucero |
Soria |
Spain |
| (26) |
Well |
Berlanga de Duero |
---------- |
Soria |
Spain |
| (27) |
Well |
FFC station |
Hortezuela |
Soria |
Spain |
| (28) |
Interstitial |
Santos Stream |
Sierra de Javalón |
Teruel |
Spain |
| (29) |
Interstitial |
Cinca Stream |
Desfiladero de la Estada |
Huesca |
Spain |
| (30) |
Interstitial |
Mijares Stream |
Montanejos |
Castellón |
Spain |
| (31) |
Interstitial |
Fardes Stream |
Lanteira |
Granada |
Spain |
| (32) |
Spring |
El Baillo |
Quesada |
Jaén |
Spain |
| (33) |
Well |
La Isla |
Arganda del Rey |
Madrid |
Spain |
| (34) |
Interstitial |
Tajuña Stream |
Luzón |
Guadalajara |
Spain |
| (35) |
Interstitial |
Tajuña Stream |
Abanades |
Guadalajara |
Spain |
| (36) |
Interstitial |
Tajuña Stream |
Loranca |
Guadalajara |
Spain |
| Hi. hortezuelensis (37) |
Well |
*FFC station |
Hortezuela |
Soria |
Spain |
Both species had previously been found in Madrid, in the Jarama River Basin in Torrelaguna, Patones, and Talamanca del Jarama
(Camacho, 1986, 1987), and Hi. mateusi also at Cueva del Reguerillo (Patones) and in other sites of the Iberian Peninsula (Soria, Guadalajara, Teruel, Toledo, Granada and Jaén, plus in Portugal (Galhano, 1967; Camacho & Serban, 2000; Camacho et al., 2000, 2014, 2017; Guil & Camacho, 2001; Camacho, 2003). Both species were recently discovered to occur at different points of the interstitial medium of the Tajuña river in the provinces of Madrid and Guadalajara (Camacho, 2019) (Table 3, Fig. 5). It is remarkable that in many occasions both species appear together. The type locality of Hi. mateusi is the hyporheic of the Duero River, 9km from the sea, near Oporto. However H. nicoleiana has not been found outside the provinces of Madrid and Guadalajara. Hexaiberobathynella is an endemic genus of the Iberian Peninsula that comprises only two species: Hi. mateusi and Hi. hortezuelensis (Soria). Hexabathynella, on the contrary, is the only cosmopolitan genus of Parabathynellidae and includes 23 species (Camacho, 2019). In the Iberian Peninsula, in addition to H. nicoleina, three more species are known to occur: H. minuta (Noodt & Galhano, 1969), found in several localities of Spain and Portugal (Table 3); H. valdecasasi Camacho, 2004 and H. sevillaensis Camacho, 2005, which are only known from their respective type localities, Arroyo el Torcón (Toledo) and Cueva de Santiago el Grande (Sevilla) respectively (Camacho, 2019) (Fig. 5).
|
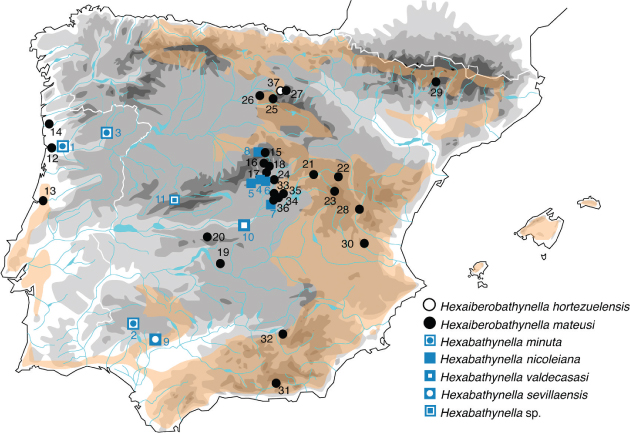 Fig. 5.— Distribution of species of Hexabathynella Schminke, 1972 and Hexaiberobathynella Camacho & Serban, 1998 genera in the Iberian Peninsula. Fig. 5.— Distribution of species of Hexabathynella Schminke, 1972 and Hexaiberobathynella Camacho & Serban, 1998 genera in the Iberian Peninsula.
Fig. 5.— Distribución de especies de los géneros Hexabathynella Schminke, 1972 y Hexaiberobathynella Camacho & Serban, 1998 en la Península Ibérica.
|
|
It is interesting to remark the mutability of some MSS habitats, that can appear as a terrestrial underground environment and, temporarily, also as an aquatic underground environment. Thus, while the flooding process occurs, the MSS may also contain species which are typically aquatic. Therefore, similarly as to in the epikarst, the same spaces can, at different times,
serve as a habitat for terrestrial and aquatic fauna, both epigean and hypogean (Ortuño et al., 2013). As seen in Figure 3, the SSD-32 collected aquatic fauna in two of the three sampling periods. In the first period, the aquatic fauna was limited to Bathynellacea (7% of the collection), and in the second period to Copepoda and Nematomorpha (50% and 3% of the collection,
respectively). During the third period, the MSS was not flooded, and only terrestrial fauna was collected.
This discovery broadens the sampling horizon for this peculiar group of aquatic crustaceans. It will be necessary to consider looking for Bathynellacea in areas where there are shallow aquifers, more or less confined, and not as deep as those that have yielded such a large number of species as in the mining arid and remote areas of Australia (Perina et al., 2018, 2019).
AcknowledgementsTOP
This work was funded by the project “Study of the diversity and distribution of the animal species of the Mesovoid Shallow Substratum in enclaves of high Mountain (Sierra de Guadarrama National Park)”, conceded by the Autonomous Organism of National Parks of Spain. Ref. (1143/2014). We would like to thank the staff at the National Park who kindly helped us with the permission applications and other formalities, and to those who also helped us with the fieldwork, especially Patricia Riquelme, Pablo Sanjuanbenito, Juan A. Vielva, Javier Donés, Marisol Redondo, Ignacio Granados, Ángel Rubio, César Martín, José Carrillo, Miguel Ángel Palomar, Ángel Velasco, Germán Mato, Manuel Criado, Enrique Calvo, Federico Madejón, Montserrat Sanz, and forestry agents of Buitrago de Lozoya. Thanks also to our colleagues who collaborated in the design of the samplings and the fieldwork, such as Alberto Jiménez Valverde, Gonzalo Pérez Suárez, Enrique Baquero, Alberto Sendra, Pablo Barranco, Alberto Tinaut, Rafael Jordana, Luis Subías, Juan José Herrero–Borgoñón, Enrique Ledesma, José D. Gilgado, Douglas Zeleppelini and Javier Ledesma.
We gratefully acknowledge C. Puch, I. Rey and A. Casado who helped us in different ways and many thanks to Damian Jaume who reviewed the English. This work has also been supported by CGL2015-66571-P, MINECO/FEDER projects.
ReferencesTOP
| ○ |
Baquero, E., Ledesma, E., Gilgado, J. D., Ortuño, V. M. & Jordana, R., 2017. Distinctive Collembola communities in the Mesovoid Shallow Substratum: First data for the Sierra de Guadarrama National Park (Central Spain) and a description of two new species of Orchesella (Entomobryidae). PLoS ONE, 12(12): e0189205. https://doi.org/10.1371/journal.pone.0189205 |
| ○ |
Camacho, A. I., 1986. A new species of the genus Hexabathynella (Syncarida, Bathynellacea, Parabathynellidae) from Spain. Bijdragen tot de Dierkunde, 56(1): 123–131. https://doi.org/10.1163/26660644-05601008 |
| ○ |
Camacho, A. I., 1987. Familia Parabathynellidae en la Península Ibérica: Taxonomía, Filogenia y Biogeografía. Tesis de Doctorado. Universidad Autónoma de Madrid, 890 pp.
|
| ○ |
Camacho, A. I., 2003. An overview of the distribution of the Parabathynellidae family (Crustacea, Syncarida, Bathynellacea)
on the Iberian Peninsula. Graellsia, 59(1): 63–78. https://doi.org/10.3989/graellsia.2003.v59.i1.224 |
| ○ |
Camacho, A. I., 2006. An annotated checklist of Syncarida (Crustacea, Malacostraca) in the world. Zootaxa, 1374: 1–54. https://doi.org/10.11646/zootaxa.1374.1.1 |
| ○ |
Camacho, A. I., 2019. Diversity, morphological homogeneity and genetic divergence in a taxonomically complex group of groundwater crustaceans: the little known Bathynellacea (Malacostraca). Bulletin de la Société d’Histoire Naturelle de Toulouse, 154: 105–160.
|
| ○ |
Camacho, A. I., Dorda, B. A. & Rey, I., 2011. Identifying cryptic speciation across groundwater populations: first COI sequences of Bathynellidae (Crustacea, Syncarida). Graellsia, 67(1): 7–12. https://doi.org/10.3989/graellsia.2011.v67.031 |
| ○ |
Camacho, A. I., Dorda, B. A. & Rey, I., 2013a. Integrating DNA and morphological taxonomy to describe a new species of the family Bathynellidae (Crustacea, Syncarida) from Spain. Graellsia, 69(2): 179–200. https://doi.org/10.3989/graellsia.2013.v69.086 |
| ○ |
Camacho, A. I., Dorda, B. A. & Rey, I., 2013b. Old and new taxonomic tools: description of a new genus and two new species of Bathynellidae from Spain with morphological and molecular characters. Journal of Natural History, 47(21–22): 1393–1420. https://doi.org/10.1080/00222933.2013.768361 |
| ○ |
Camacho, A. I., Dorda, B. A. & Rey, I., 2014. Iberian Peninsula and Balearic Island Bathynellacea (Crustacea, Syncarida) database.
ZooKeys, 386: 1–20. https://doi.org/10.3897/zookeys.386.6296 |
| ○ |
Camacho, A. I., Mas-Peinado, P., Watiroyram, S., Brancelj, A., Bandari, E., Dorda, B. A., Casado, A. & Rey, I., 2018. Three new species of Bathynellacea (Crustacea Malacostraca) from Thai Caves. Preliminar global molecular phylogeny of the family Parabathynellidae. Contributions to Zoology, 87(4): 227–260. https://doi.org/10.1163/18759866-08704002 |
| ○ |
Camacho, A. I., Sánchez-Chillón, B., Dorda, B. A. & Rey, I., 2017. The collection of Bathynellacea specimens of MNCN (CSIC)
Madrid: microscope slides and DNA extract. ZooKeys, 678: 31–36. https://doi.org/10.3897/zookeys.678.11543 |
| ○ |
Camacho, A. I. & Serban, E., 2000. Revisión del grupo Iberobathynella (Iberobathynella) Camacho & Serban, 1998 (Crustacea, Syncarida, Parabathynellidae) endémico de la Península Ibérica. Graellsia, 56: 35–48. https://doi.org/10.3989/graellsia.2000.v56.i0.308 |
| ○ |
Camacho, A. I., Serban, E. & Guil, N., 2000. Phylogenetical review and biogeographical remarks on the interstitial and subterranean freshwater iberobathynells (Crustacea, Syncarida, Parabathynellidae) in Spain. Journal of Natural History, 34: 563–585. https://doi.org/10.1080/002229300299444 |
| ○ |
Galhano, M. E., 1967. Sur une nouvelle Parabathynella psammique du Portugal. Publicaçoes do Instituto de Zoologia “Dr. Augusto Nobre”, 98: 9–18.
|
| ○ |
Gers, C., 1992. Ecologie et biologie des Arthropodes Terrestres du Milieu Souterrain Superficiel. Fonctionnement et Ecologie Evolutive. DPhil Thesis, Université Paul Sabatier de Toulouse.
|
| ○ |
Guil, N. & Camacho, A. I., 2001. Historical biogeography of Iberobathynella (Crustacea, Syncarida Parabathynellidae) an aquatic subterranean genus of bathynellids, endemic to Iberian Peninsula. Global Ecology and Biogeography, 10: 487–501.
|
| ○ |
Juberthie, C., Bouillon, M. & Delay, B., 1981. Sur l’existence du milieu souterrain superficiel en zone calcaire. Mémoires de Biospéologie, 8: 77–93.
|
| ○ |
Juberthie, C. & Decu, V., 1994. Structure et diversité du domaine souterrain: particularités des habitats et adaptations des espèces. In: Juberthie, C. & Decu. V. (eds.). Encyclopaedia biospeologica. Tome I. Société Internationale de Biospéologie. Moulis: 5–22.
|
| ○ |
Juberthie, C., Delay, B. & Bouillon, M., 1980. Extension du milieu souterrain en zone non-calcaire: description d’un nouveau milieu et de son peuplement par les coléoptères troglobies. Mémoires de Biospéologie, 7: 19–52.
|
| ○ |
Ledesma, E., Jiménez-Valverde, A., de Castro, A., Aguado-Aranda, P. & Ortuño, V., 2019. The study of hidden habitats sheds light on poorly known taxa: spiders of the Mesovoid Shallow Substratum. ZooKeys, 841: 39–59. https://doi.org/10.3897/zookeys.841.33271 |
| ○ |
López, H. & Oromí, P., 2010. A type of trap for sampling the mesovoid shallow substratum (MSS) fauna. Speleobiology Notes, 2: 7–11.
|
| ○ |
Mammola, S., Giachino, P. M., Piano, E., Jones, A., Barberis, M., Badino, G. & Isaia, M., 2016. Ecology and sampling techniques of an understudied subterranean habitat: the Milieu Souterrain Superficiel (MSS). The Science of Nature, 103(11–12): 88. https://doi.org/10.1007/s00114-016-1413-9 |
| ○ |
Medina, A. L. & Oromí, P., 1990. First data on the superficial underground compartment in La Gomera (Canary Islands). Mémoires de Biospéologie, 17: 87–91.
|
| ○ |
Mestrov, M., 1962. Un nouveau milieu aquatic souterrain: le biotope hypotelminorhéique. Compte Rendus de Académie des Sciences, 254: 2677–2679.
|
| ○ |
Ortuño, V. M., Gilgado, J. D., Jiménez-Valverde, A., Sendra, A., Pérez–Suárez, G. & Herrero–Borgoñón, J. J., 2013. The “Alluvial Mesovoid Shallow Substratum”, a new subterranean habitat. PLoS ONE, 8(10): e76311. https://doi.org/10.1371/journal.pone.0076311 |
| ○ |
Ortuño, V. M., Ledesma, E., Jiménez-Valverde, A. & Pérez-Suárez, G., 2019. Studies of the mesovoid shallow substratum can change the accepted autecology of species: the case of ground beetles (Coleoptera: Carabidae) in the Sierra de Guadarrama National Park (Spain). Animal Biodiversity and Conservation, 42(2): 213–226. https://doi.org/10.32800/abc.2019.42.0213 |
| ○ |
Perina, G. & Camacho, A. I., 2016. Permanent slides for morphological studies of small crustaceans: Serban’s method and its variation applied on Bathynellacea (Malacostraca). Crustaceana, 89(10): 1161–1173. http://dx.doi.org/10.1163/15685403-00003576 |
| ○ |
Perina, G., Camacho, A. I., Huey, J., Hoewitz, P. & Koenders, A., 2018. Understanding subterranean variability: the first genus of Bathynellidae (Bathynellacea, Crustacea) from Western Australia described through a morphological and multigene approach.
Invertebrate Systematics, 32: 423–447. https://doi.org/10.1071/IS17004 |
| ○ |
Perina, G., Camacho, A. I., Huey, J., Hoewitz, P. & Koenders, A., 2019. New Bathynellidae (Crustacea) taxa and their relationships in the Fortescue catchment aquifers of the Pilbara region, Western Australia. Systematics and Biodiversity, 17(2): 148–164. https://doi.org/10.1080/14772000.2018.1559892 |
| ○ |
Pipan, T. & Culver, D., 2012. Convergence and divergente in the subterranean realm: a reassessment. Biological Journal of the Linnean Society, 107: 1–14. https://doi.org/10.1111/j.1095-8312.2012.01964.x |
| ○ |
Pipan, T., López, H., Oromí, P., Polak, S. & Culver, D. C., 2011. Temperature variation and the presence of troglobionts in shallow subterranean habitats. Journal of Natural History, 45: 253–273. https://doi.org/10.1080/00222933.2010.523797 |
| ○ |
Ruzicka, V., Hager, J. & Zacharda, M., 1995. Arachnid population patterns in undergoround cavities of a stony debris field
(Araneae, Opiliones, Psudoscorpionidea, Acari: Prostigmata, Rhagidiidae). Pedobiologia, 39: 42–51.
|
| ○ |
Uéno, S. I., 1980. The anophthalmic trechine beetles of the group of Trechiama ohshimai. Bulletin of the National Museum of Nature and Science, Series A, Zoology, 6: 195–274.
|
| ○ |
Uéno, S. I., 1981. New anophthalmic Trechiama (Coleoptera, Trechinae) from northern Shikoku, Japan. Journal of the Speleological Society of Japan, 6: 11–18.
|
| ○ |
Vialette, Y., Casquet Fúster, J. M., Ibarrola, E., Navidad, M., Peinado, M. & Villaseca, C., 1987. Geochronological study of orthogneisses from the Sierra de Guadarrama (Spanish Central System). Neues Jahrb Mineral Monatsh, 10: 465–479.
|
Fig. 1.— Distribution of subterranean sampling devices in the Sierra de Guadarrama National Park (Madrid, Spain) and (in red Bathynellacea specimens found).