NON-ALLOMETRIC VARIATION IN THE BROWN-THROATED SLOTH BRADYPUS VARIEGATUS (SCHINZ, 1825) SKULL (MAMMALIA, PILOSA, BRADYPODIDAE)
P. M. Parés-Casanova
Department of Animal Science, University of Lleida, Av. Rovira Roure 191, E-25198, Catalonia, Spain. Email: peremiquelp@ca.udl.cat – http://orcid.org/0000-0003-1440-6418
| |
ABSTRACT
Brown-throated sloth Bradypus variegatus (Schinz, 1825) is a monomorphic mammal, and its skull ontogeny is poorly known. Here, we present a study of the ontogenetic allometric relationship between skull size and shape in 21 specimens of different sizes, for which size and shape were determined by means of geometric morphometric methods. Results indicate that skull shape variation can hardly be explained by skull size. Several studies have shown unique morphological traits of sloths from mammalian norms, affecting varied phenotypic traits from skeletal parts to soft tissues. This non-allometric scaling of skull form in sloth can be seen as another uniqueness of this taxonomic group.
Keywords: allometry; Choloepus; three-toed sloth; two-toed sloth; Xenarthra.
|
| |
RESUMEN
Variación no alométrica en el cráneo del perezoso bayo Bradypus variegatus (Schinz, 1825) (Mammalia, Pilosa, Bradypodidae)
El perezoso bayo Bradypus variegatus (Schinz, 1825) es un mamífero monomórfico, de ontogenia craneal poco conocida. En este estudio analizamos la alometría estática entre tamaño y forma, utilizando 21 especímenes diferentes de edades diversas. El tamaño y la forma fueron determinados mediante técnicas de morfometría geométrica. De los resultados obtenidos se desprende que la variación en la forma craneal queda muy poco explicada por la variación en el tamaño. Muchos estudios han señalado características morfológicas únicas en los perezosos en relación al resto de mamíferos, características fenotípicas que van de la estructura esquelética a tejidos blandos. En este caso, el escalado no alométrico del cráneo debería ser visto como otra característica única de este grupo taxonómico.
Palabras clave: alometría; Choloepus; perezoso de tres dedos; perezoso de dos dedos; Xenarthra.
|
IntroductionTOP
Sloths present some anatomical features which are unique in mammals (Hautier et al., 2014; Galliari & Carlini, 2018), such as the variation in the number of cervical vertebrae the specific sequence of cranial suture closure (Rager et al., 2014; Montilla-Rodríguez et al., 2016), the synsacral number (Montilla-Rodríguez et al., 2016; Galliari & Carlini, 2018), the early ossification of the pubis and the phalanges and late ossification of the sternum (Hautier et al., 2011; Tague, 2019), architectural properties of forelimb muscles (Olson et al., 2018). With no deciduous teeth, they have a single set of high-crowned, open-rooted teeth, which grow continuously throughout life. Incisors are absent, and it is not really possible to distinguish between premolars and molars in the cheek series, which are quite similar (Hautier et al., 2016).
Sloths are included in the superorder Xenarthra Cope, 1889. Xenarthra is a mammalian group that includes a wide variety of locomotions and lifestyles, including the digging armadillos and anteaters, completely arboreal modern sloths and extinct terrestrial giant ground sloths (Hautier et al., 2014). They are grouped together with anteaters in the order Pilosa Flower, 1883 (Hautier et al., 2014). Extant sloths consist of only two genera, Choloepus Illiger, 1811 and Bradypus Linnaeus, 1758 (Hautier et al., 2014). One of their differences is found in the number of fingers. Three-fingered genus Bradypus comprises four extant species: B. tridactylus Linnaeus, 1758, B. torquatus Illiger, 1811, B. variegatus Schinz, 1825, and B. pygmaeus Anderson & Handley, 2001. Bradypus is native to the rainforests of South America where, as arboreal herbivores, they spend much of their time in the tree tops eating leaves and fruit (Sampedro-Marín et al., 2018). The head is the centre of the primary sense organs, with outer ears (pinnae) being tiny and hardly visible on the head and eyes having the possibility to retract in their sockets. Both of these characteristics reveal externally a partially muted function of the head within the whole animal (Montilla-Rodríguez et al., 2016).
Despite the extensive literature on the ecology and behaviour related to sloths, research on their developmental basis, and particularly centred on the skull, is rare. Cartelle & De Iuliis (2006) studied the skull ontogeny in the extinct Eremotherium laurillardi (Lund, 1842), the Pan-American giant ground sloth. Hautier et al. (2014) explored the patterns of the intra- and interspecific morphological variation of the skull. Riesco López & Bastir (n.d.) studied virtually the morphology of extincted Megatherium americanum Cuvier, 1796. In an ancient publication, Parker (1885) stated only that sloth skulls grow greatly from birth to full adulthood. In the complete sloths monography by Naples (1989), she does not focus on allometry.
Allometry is the statistical association between size and shape (Mosimann, 1970). In an extended meaning, allometry can be understood as differential change of a quantitative character with variation in overall body size. According to Klingenberg, ontogenetic allometry (or growth allometry, changes of shape versus size) deals with covariation among anatomical features during growth (Klingenberg & Zimmermann, 1992). Static allometry, on the other hand, denotes size-related shape changes, measured in different individuals at the same developmental stage within a population or species. Allometric relationships are important sources of information for many types of biological research. The baseline for an allometric relationship is isometry (or geometric similarity), i.e. that shape is invariant of size. This is why, here, we explore skull allometry in the brown-throated sloth Bradypus variegatus. Thus, this work tries to contribute to the knowledge of ontogenetic skull growth in sloths, based on a geometric morphometrics new approach.
Material and methodsTOP
SAMPLESTOP
We examined the complete collection of 21 entire skulls of Bradypus variegatus archived in the collections of the Departamento de Biología of the Universidad del Valle in Cali (Colombia) and Instituto de Ciencias Naturales of the Universidad Nacional de Colombia. Every specimen had been taxonomically identified to the species level, and was initially collected for other studies. Although there is a certain body sexual dimorphism present in B. variegatus (Hautier et al., 2014) we performed all our analyses regardless of gender (moreover, this data were lacking in most of the individuals studied). All specimens had been collected wild from different places in Colombia. No information on age was available. Individuals ranged from 45.5 to 77.1 mm total length, and it can be considered that they represent an ontogenetic sequence from juveniles to sexually mature adults.
TAKING PHOTOGRAPHS AND DIGITIZINGTOP
Digital images of skull ventral aspects were taken with a Nikon D1500 digital camera equipped with an 18-105 mm Nikon DX telephoto lens. Each skull was placed in the centre of the optical field, with its ventral face oriented parallel to the image plane. A set of 18 landmarks (Table 1) were digitized using TpsDig v. 2.16 software (Rohlf, 2015a). The landmarks chosen were present on all specimens and, having biological significance, were considered by us to sufficiently capture the morphology of the splanchno and neurocranium on their ventral aspect (Montilla-Rodríguez et al., 2016) (Fig. 1). Shape alignment was achieved with generalized Procrustes analysis in the package MorphoJ (Klingenberg, 2011). This method is an iterative procedure that scales, rotates and translates landmarks to reduce the sum of squared distances of specimen landmarks to an average shape (Adams et al., 2013). Landmarks were digitized twice and two replicas compared to assess bilateral asymmetries using a Procrustes ANOVA using an isotropic model. All measurements were taken by the same author to reduce experimenter effect. The Procrustes distance between a shape and its reflection is a measure of asymmetry, being 0 only for perfectly symmetric shapes. There is no directional asymmetry when left-right differences of Procrustes distances from the reference shape are normally distributed with a mean of 0. Fluctuating asymmetry was assessed as “individual * side” interaction while directional asymmetry as “side” factor. This analysis were done with MorphoJ software (Klingenberg, 2011).
Table 1.—Set of landmarks (18) used in this study.
Tabla 1.— Conjunto de hitos (18) utilizados para esta investigación.
| Most rostral midline part of splanchnocranium |
| Most caudal midline part of hard palate |
| Central occlusal aspect for each upper tooth (1 canine and 4 molars) |
| Most lateral part of squamous apophysis of temporal bone |
| Condilar foramen |
| Most rostral midline part of foramen magnum |
| Most caudal midline part of foramen magnum |
|
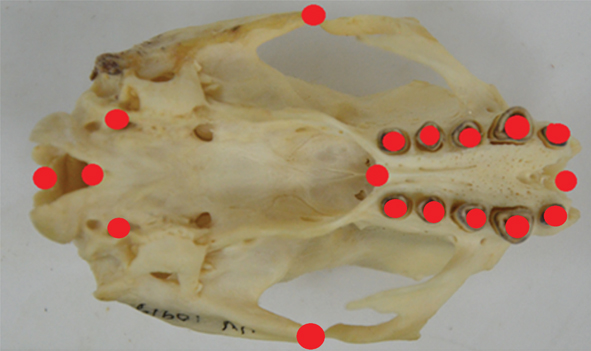 Fig. 1.— Ventral skull view of Bradypus variegatus. Eighteen landmarks (four of them on the midline and the rest paired) were used to capture skull shape. Skulls were aligned by their dorsal plane to a stable plane. Fig. 1.— Ventral skull view of Bradypus variegatus. Eighteen landmarks (four of them on the midline and the rest paired) were used to capture skull shape. Skulls were aligned by their dorsal plane to a stable plane.
Fig. 1.— Vista ventral del cráneo de Bradypus variegatus. Fueron utilizados 18 hitos (4 de ellos en la línea media y el resto pareados) para capturar la forma craneal. Los cráneos fueron colocados sobre su cara dorsal en una superficie estable)
|
|
STATISTICAL ANALYSISTOP
TpsSmall v. 1.29 software was used to assess the correlation between the Procrustes and tangent shape distances (Rohlf, 2015b). To obtain information on shape variation related to size, with position and orientation, the data were first superimposed on Bookstein’s shape coordinates and Centroid size (CS) was obtained. CS is the square root of the summed squared distances of each landmark from the centroid of the landmark configuration, and was interpreted as the geometric measure of size (Rohlf & Bookstein, 1990; Mitteroecker et al., 2013). MorphoJ software (Klingenberg, 2011) was used for this analysis.
SYMMETRIZINGTOP
In a previous analysis, it was proved that there was no directional asymmetry in the anatomical landmarks (p = 0.424) studied but there was some fluctuating bilateral asymmetry (p < 0.0001). Fluctuating asymmetry can be defined as the asymmetry due to chance fluctuation in the development of the left and right sides of the body or organs (Mehmet, 2008). To correct for this asymmetry, a computation based on a Procrustes superimposition of the landmark configurations with the reflection of the skull was used (Yazdi, 2014).
CENTROID SIZETOP
Centroid size (CS) is a measure of overall size. It is the square root of the summed squared distances of each landmark from the median shape of the landmark configuration (Bookstein, 1991). In the absence of allometry CS is the only size measurement that is uncorrelated with shape variation (Yazdi, 2014). CS was calculated from the raw coordinates (before symmetrizing) of the landmarks (Yazdi, 2014) and tested for normality using the Shapiro-Wilk test using the PAST software .
ANALYSIS OF ALLOMETRYTOP
There were no specimens of accurately known age available, but being neither anatomical features (cranial, postcranial) to differentiate ontogenetic stages in skulls nor post-cranial bones associated to these skulls, growth (no age) was studied according to skull size (expressed as CS). So we regressed shape on log-CS to obtain allometric relationship. CS is commonly used to investigate allometry in morphometric studies (Adams et al., 2013). The percent of shape variation accounted for by regression with log-CS was used as an indicator of the relative strength of the relationship (Adams et al., 2013). The software MorphoJ (Klingenberg, 2011) was used for this analysis.
ResultsTOP
PREVIOUS ANALYSISTOP
The correlation was very close to linear for all of the data (r = 0.999; slope, b = 0.995, and therefore no specimens deviated appreciably from the linear regression line), suggesting that tangent space was an adequate approximation to Kendall. Thus, although the ventral aspect of the skull is not a perfect flat object, the authors considered that the two-dimensional approach applied implies a limited loss of information, and we proceeded with the morphometric analyses. The averaged error of skull CS did not exceed 0.09% of the total variation (F = 0.00019, p = 0.988), and p = 0.999 for skull shape variables (p-value from permutation tests, 10,000 permutation rounds). This means that the measurement error explained a negligible percentage of variance.
CENTROID SIZETOP
CS presented a range of 412.42 to 667.34, giving a clear idea of the skull size variation. The distribution of CS did not differ significantly from normal (W = 0.976, p = 0.863).
RELATIONSHIP BETWEEN SIZE AND SHAPETOP
The results of multivariate regression of shape variables on log CS revealed that only a 3.27% of shape variation was explained by size (p = 0.797) (Fig. 2). The null hypothesis of isometry was accepted (allometry was not present) in all anatomical landmarks since regression was not statistically significant.
|
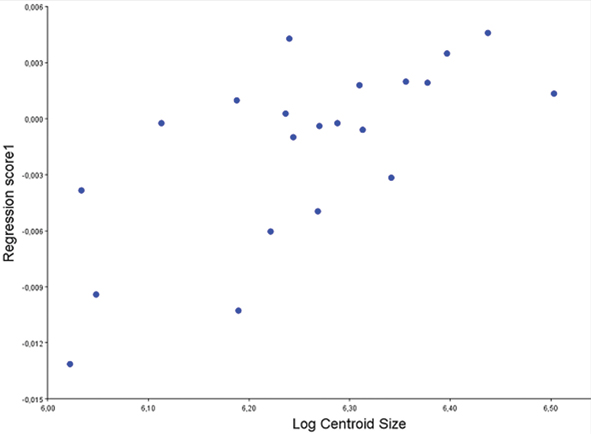 Fig. 2.— Multivariate regression of shape variables (Y-axis) on log of Centroid Size (X-axis) for 21 skulls of Bradypus variegatus. This revealed that shape variation could hardly be explained by size (p = 0.797). The null hypothesis of isometry was then accepted (allometry was not present) in all anatomical landmarks. Fig. 2.— Multivariate regression of shape variables (Y-axis) on log of Centroid Size (X-axis) for 21 skulls of Bradypus variegatus. This revealed that shape variation could hardly be explained by size (p = 0.797). The null hypothesis of isometry was then accepted (allometry was not present) in all anatomical landmarks.
Fig. 2.— Regresión multivariada de las variables de tamaño (eje Y) sobre el tamaño del centroide (eje X) para 21 cráneos de Bradypus variegatus. Este análisis demostró que la variación de la forma se explicaba escasamente por el tamaño. La hipótesis nula fue pues aceptada (la alometría no estaba presente) para todos los hitos anatómicos (p = 0.797).
|
|
DiscussionTOP
Variation of the specimens in shape space was perfectly correlated with tangent space for all anatomical landmarks. This allowed us the use of the plane approximation in statistical analysis and interpretation of results.
Modifications of patterns of ontogenetic allometry have been shown to affect the magnitude of morphological change in many clades (Wilson, 2018), although it has been frequently underappreciated. Geometrical similarity in B. variegatus skull is maintained with size increase, e.g. skull development is isometric. Constant area-to-volume ratios, an adaptive necessity for many organic relationships, can only be maintained by altering shape. However, in sloths, growth produces individuals of no detectably different form, at least along the allometric range studied, e.g. they preserve the shape with no differential changes of anatomical points. With growth having no significant effect on skull shape, the size of an animal does not determine different mechanical functions; in other words, adult individuals are only a scaled version of juveniles These mechanical cranial functions, which are the same throughout the animal’s life, are probably related to arboreal behaviour (for instance, neck and trunk muscles inserting on skull, skull muscles and the connecting ligament system, frontal sinus, and upper cheek teeth). More research is, however, needed to elucidate such functional aspects derived from skull isometry.
Scaling predictions state that isometric changes in kinematics result from isometric changes in size. This prediction is difficult to support in B. variegatus. In any case, the non-geometric scaling of skull form that has been stated in this research can be seen as another uniqueness of sloths. It would now be interesting to test whether this “algebraic identity of ontogeny” (the maintenance of geometrical similarity with size increase) could also have different phylogenetic differences in the superorder Xenarthra skulls. Another benefit can arise from this general conclusion. Although sloths show great diversity in the combination of their cranial characters, both within and between the three fossil families and the family of recent tree sloths, if we had the ability to reconstruct morphometric data for key fossil taxa, their integration into comparative phylogenetic analyses would be of paramount importance to understand the evolution of body size.
AcknowledgementsTOP
We thank Catalina Cárdenas and Hugo Fernando López for access to the Instituto de Ciencias Naturales of the Universidad Nacional de Colombia collection, and Óscar Murillo from Departamento de Biología of the Universidad del Valle in Cali. We also thank reviewers for their comments and suggestions. No financial assistance was received.
ReferencesTOP
| ○ |
Adams, D. C., Rohlf, F. J. & Slice, D. E., 2013. A field comes of age: geometric morphometrics in the 21st century. Hystrix, 24(1): 7–14. https://doi.org/10.4404/hystrix-24.1-6283 |
| ○ |
Bookstein, F. L., 1991. Morphometric Tools for Landmark Data. Geometry and Biology. Cambridge University Press. Cambridge. |
| ○ |
Cartelle, C. & De Iuliis, G., 2006. Eremotherium Laurillardi (Lund) (Xenarthra, Megatheriidae), the Panamerican giant ground sloth: Taxonomic aspects of the ontogeny of skull and dentition. Journal of Systematic Palaeontology, 4(2): 199-209. https://doi.org/10.1017/S1477201905001781 |
| ○ |
Galliari, F. C. & Carlini, A. A., 2018. Xenarthran synsacrum morphology and evolution. Journal of Mammalian Evolution. https://doi.org/10.1007/s10914-018-9442-0 |
| ○ |
Hammer, Ø., Harper, D. A. T. & Ryan, P. D., 2001. PAST v. 2.17c. Palaeontologia Electronica, 4(1): 1–229. |
| ○ |
Hautier, L., Billet, G., Eastwood, B. & Lane, J., 2014. Patterns of Morphological Variation of Extant Sloth Skulls and their Implication for Future Conservation Efforts. Anatomical Record, 297(6): 979–1008. https://doi.org/10.1002/ar.22916 |
| ○ |
Hautier, L., Gomes Rodrigues, H., Billet, G. & Asher, R. J., 2016. The hidden teeth of sloths: Evolutionary vestiges and the development of a simplified dentition. Scientific Reports, 6: 1–9. https://doi.org/10.1038/srep27763 |
| ○ |
Hautier, L., Lebrun, R., Saksiri, S., Michaux, J., Vianey-Liaud, M. & Marivaux, L., 2011. Hystricognathy vs. sciurognathy in the rodent jaw: a new morphometric assessment of hystricognathy applied to living fossil Laonastes (Rodentia, Diatomyidae). PLoS ONE, 6(4): e18698. https://doi.org/10.1371/journal.pone.0018698 |
| ○ |
Klingenberg, C. P., 2011. MorphoJ: An integrated software package for geometric morphometrics. Molecular Ecology Resources, 11(2): 353–357. https://doi.org/10.1111/j.1755-0998.2010.02924.x |
| ○ |
Klingenberg, C. P., & Zimmermann, M., 1992. Static, ontogenetic, and evolutionary allometry: a multivariate comparison in nine species of water striders. The American Naturalist, 140(4): 601–619. https://doi.org/10.1086/285430 |
| ○ |
Mehmet, M., 2008. Asymmetry measures and allometric growth parameter estimates for investigate effect of early feed restriction on deviation from bilateral symmetry in broiler chickens. Archiv für Tierzucht, 6: 611–619. https://doi.org/10.5194/aab-51-611-2008 |
| ○ |
Mitteroecker, P., Gunz, P., Windhager, S. & Schaefer, K., 2013. A brief review of shape, form, and allometry in geometric morphometrics, with applications to human facial morphology. Hystrix, 24(1): 59–66. https://doi.org/10.4404/hystrix-24.1-6369 |
| ○ |
Montilla-Rodríguez, M. A., Blanco-Rodríguez, J. C., Nastar-Ceballos, R. N. & Muñoz-Martínez, L. J., 2016. Descripción anatómica de Bradypus variegatus en la Amazonia colombiana (Estudio preliminar). Revista de la Facultad de Ciencias Veterinarias, 57(1): 3–14. |
| ○ |
Mosimann, J. E., 1970. Size allometry: size and shape variables with characterizations of the lognormal and generalized gamma distribution. Journal of the American Statistical Association, 65(330): 930–945. |
| ○ |
Naples, V., 1989. The feeding mechanism in the Pleistocene ground sloth, Glossotherium. Contributions in Science, 415: 1–23. |
| ○ |
Olson, R. A., Glenn, Z. D., Cliffe, R. N. & Butcher, M. T., 2018. Architectural properties of sloth forelimb muscles (Pilosa: Bradypodidae). Journal of Mammalian Evolution, 25(4): 573–588. https://doi.org/10.1007/s10914-017-9411-z |
| ○ |
Parker, W. K., 1885. On the structure and development of the skull in Mammalia Part II. Edentata. Philosophical Transactions of the Royal Society of London, 176: 1–119. https://doi.org/10.1098/rstl.1885.0001 |
| ○ |
Rager, L., Hautier, L., Forasiepi, A., Goswami, A. & Sánchez-Villagra, M. R., 2014. Timing of cranial suture closure in placental mammals: Phylogenetic patterns, intraspecific variation, and comparison with marsupials. Journal of Morphology, 275(2): 125–140. https://doi.org/10.1002/jmor.20203 |
| ○ |
Riesco López, A. & Bastir, M. (n.d.). Morfología virtual en 3D del Megatherium americanum del MNCN. Retrieved from https://digital.csic.es/bitstream/10261/147395/20/Riesco Lopez.pdf |
| ○ |
Rohlf, F. J., 2015a. The tps series of software. Hystrix, 26(1): 9–12. https://doi.org/10.4404/hystrix-26.1-11264 |
| ○ |
Rohlf, F. J., 2015b. TpsSmall v. 1.33. Retrieved from http://life.bio.sunysb.edu/morph/ |
| ○ |
Rohlf, F. J. & Bookstein, F. L. (Eds.), 1990. Proceedings of the Michigan Morphometrics Workshop. Special Publication No. 2. The University of Michigan Museum of Zoology. Ann Arbor. 396 pp.
|
| ○ |
Sampedro-Marín, A., Aguas-Montes, K. & Jiménez-Pineda, D., 2018. Estado de conservacion y caracterización del hábitat de Bradypus variegatus Schinz, 1825 (Mammalia: Xenarthra) durante la época seca, en el departamento de Sucre, Colombia. Revista Colombiana de Ciencia Animal - RECIA, 3(1): 15. https://doi.org/10.24188/recia.v3.n1.2011.247 |
| ○ |
Tague, R. G., 2019. Commonality in pelvic anatomy among three fossorial, scratch-digging, mammalian species. Journal of Mammalian Evolution. https://doi.org/10.1007/s10914-019-09463-y |
| ○ |
Wilson, L. A. B., 2018. The evolution of ontogenetic allometric trajectories in mammalian domestication. Evolution, 72(4): 867–877. https://doi.org/10.1111/evo.13464 |
| ○ |
Yazdi, A. B., 2014. Application of geometric morphometrics to analyse allometry in two species of the genus Myrmica (Hymenoptera: Formicidae). Soil Organisms, 86(1): 77–84. Retrieved from http://www.senckenberg.de/files/content/forschung/publikationen/soilorganisms/volume_86_1/4_86-1-05_bagherian.pdf |
Fig. 1.— Ventral skull view of Bradypus variegatus. Eighteen landmarks (four of them on the midline and the rest paired) were used to capture skull shape. Skulls were aligned by their dorsal plane to a stable plane.