EUPELMIDAE (HYMENOPTERA, CHALCIDOIDEA) OF IBERIA AND THE CANARY ISLANDS: AN ANNOTATED CHECKLIST WITH DESCRIPTIONS OF SOME PREVIOUSLY UNRECOGNISED MALES AND A NEW SPECIES OF CALOSOTA CURTIS, 1836
R. R. Askew 1 & J. L. Nieves-Aldrey 2
1 Le Bourg, St Marcel du Périgord, 24510 Ste Alvère, France. ORCID iD: http://orcid.org/0000-0002-3860-3697. E-mail: olynx@btinternet.com
2 Museo Nacional de Ciencias Naturales (CSIC), Departamento de Biodiversidad y Biología Evolutiva, José Gutiérrez Abascal 2, ES-28006 Madrid, España. ORCID iD: http://orcid.org//0000-0002-4711-7455. E-mail: aldrey@mncn.csic.es (corresponding author)
| |
ABSTRACT
The eighty-four species of Eupelmidae known from the Iberian Peninsula and Canary Islands are listed with distributional data and host records. A new species of Calosota, C. carmenae Askew & Nieves-Aldrey sp. n. is described. The previously unrecognised males of Calosota bolivari Askew, 2006, C. nitens Askew, 2006, Anastatus oscari (Ruthe, 1859) and A. uromeni Ferrière, 1968 are also described. Neanastatus africanus Ferrière, 1938, Eupelmus confusus Al khatib, 2015, E. gemellus Al khatib, 2015, E. kiefferi De Stefani, 1898, E. martellii Masi, 1941 and E. stramineipes Nikol’skaya, 1952 are added to the list of Spanish Eupelmidae, and E. fulvipes Förster, 1860 is removed.
urn:lsid:zoobank.org:pub:A0A4215E-6718-4185-9C08-CFD22F51A2BD
Key words: Hymenoptera; Chalcidoidea; Eupelmidae; Spain; Portugal; Canary Islands; Calosota; new species.
|
| |
RESUMEN
Eupelmidae de Iberia y las Islas Canarias: Check list comentada de las especies, incluyendo la descripción de una nueva especie de Calosota Curtis, 1836 y de los machos previamente no reconocidos de algunas especies
Se presenta un listado taxonómico comentado de las ochenta y cuatro especies de Eupelmidae que se conocen de la Península Ibérica e Islas Baleares, incluyendo nuevos datos de distribución y de especies hospedadoras. Se describe una nueva especie de Calosota, C. carmenae Askew & Nieves-Aldrey sp. n. Se describen también los machos, hasta ahora no reconocidos, de cuatro especies: Calosota bolivari Askew, 2006, C. nitens Askew, 2006, Anastatus oscari (Ruthe, 1859) and A. uromeni Ferrière, 1968. Neanastatus africanus Ferrière, 1938, Eupelmus confusus Al khatib, 2015, E. gemellus Al khatib, 2015, E. kiefferi De Stefani, 1898, E. martellii Masi, 1941 y E. stramineipes Nikol’skaya, 1951 se incorporan al listado de Eupelmidae de España, mientras que E. fulvipes Förster, 1860 se elimina de dicha lista.
Palabras clave: Hymenoptera; Chalcidoidea; Eupelmidae; España; Portugal; Islas Canarias; Calosota; especie nueva.
|
IntroductionTOP
Recent taxonomic works on Eupelmidae prompt us to review the fauna of Iberia and the Canary Islands as presented in Askew & Nieves-Aldrey (2000, 2004, 2006). The large genus Eupelmus Dalman, and the E. urozonus Dalman species complex, have been revised respectively by Gibson & Fusu (2016) and Al khatib et al. (2014, 2015), and species of Eupelmus in subgenus Macroneura Walker are the subject of continuing research by Lucian Fusu. European species of Calosota Curtis were reviewed, and Nearctic species revised, by Gibson (2010). Palaearctic species of Reikosiella Yoshimoto were revised by Fusu (2013) and this name was synonymised under Merostenus Walker by Gibson (2017). In addition, important new data on Eupelmidae were provided by the sadly all too brief, but nevertheless extremely productive, activity of the late Antoni Ribes at various locations in the Catalonian province of Lleida (Lérida), and by Malaise trap sampling in 2013 by Carmen Rey at La Parata, Mojácar, in the Andalucian province of Almería. These and other studies have added 24 species and one genus to the fauna list, and deleted one species. Eighty-four species are now known from the Iberian Peninsula and Canary Islands, 39 Eupelmus, 10 each of Calosota and Anastatus Motschulsky, 7 Eusandalum Ratzeburg, 5 Merostenus Walker, 4 Calymmochilus Masi, 2 each of Neanastatus Girault, Brasema Cameron and Arachnophaga Ashmead, and 1 each of Pentacladia Westwood, Metapelma Westwood and Tineobius Ashmead. A new species of Calosota and the newly recognised males of two Calosota and two Anastatus species are described. New distributional and biological data for previously recorded species are presented.
The dates of publication of two papers by Bolívar y Pieltain (1934, 1935) as quoted in Askew & Nieves Aldrey (2000, 2004), require correction. Descriptions of Eupelmus juniperinus, E. moroderi and Arachnophaga matritensis were published in 1934 (not 1933) and that of Anastatus catalonicus in 1935 (not 1934).
Abbreviations used as follows: AR = Antoni Ribes, BMNH = The Natural History Museum (London), CR = Carmen Rey, JLNA = J. L. Nieves-Aldrey, MNCN = Museo Nacional de Ciencias Naturales (Madrid), RMNH = Rijksmuseum van Natuurlijke Historie (Leiden), RRA = R. R. Askew.
EUPELMIDAE OF SPAIN, PORTUGAL AND THE CANARY ISLANDSTOP
The following is an updated review of all Eupelmidae recorded from the region. Species additional to those listed in Askew & Nieves-Aldrey (2000, 2004, 2006) are indicated by a dagger (†). Genera and species are arranged alphabetically within subfamilies.
CALOSOTINAETOP
Calosota Curtis, 1836
Calosota aestivalis Curtis, 1836
No records additional to those given in Askew & Nieves-Aldrey (2006) are available, but see comments under C. bolivari.
Calosota ariasi Bolívar y Pieltain, 1929
Calosota ariasi may be a junior synonym of C. acron (Walker, 1848) (Askew & Nieves-Aldrey, 2006) or of C. aestivalis (Gibson, 2010).
Calosota bolivari Askew, 2006
A female of C. bolivari in MNCN, misidentified as C. dusmeti Bolívar y Pieltain, 1929 (Askew & Nieves-Aldrey, 2006) and collected with the holotype female of C. bolivari (Madrid, El Ventorrillo, Malaise trap, 14.vii.1991, A. Garrido), has been determined as C. bolivari by Gibson (2010). This specimen has a yellow scape as in C. dusmeti, but unlike the holotype of C. bolivari, and this led to the misidentification. Another female was found in a Malaise trap sample, Almería, Mojácar, 21.vii-3.viii.2013, CR. Gibson (2010) suggests the possible synonymy of C. bolivari under C. agrili Nikol’skaya, 1952.
Gibson (2010) identified a series of 14 male C. bolivari in MNCN that had been collected by Malaise trap in 1988 and 1989 by JLNA and CR at the type locality (El Ventorrillo, altitude 1480m), plus a single male (described below) with the same collection data as the female holotype.
MALE. Length 2.5 mm. Head purple-black, frons blue-green; antennal scape dark, metallic. Thoracic dorsum dark green, the scutellum slightly darker than the mesoscutum. Side of thorax mostly dark green but the mesepimeron posteriorly purplish black. Propodeum dark green. Tegula and fore wing venation brown; wing setation dark and easily visible. Legs dark, femora and tibiae yellow-brown only at extreme apices, meso- and metatarsi with basal three segments paler than brown apical segments. Gaster with dorsal surface violet-black.
Differs structurally from female particularly in the form of antenna and gaster. Vertex with quite strongly raised reticulate sculpture. Antenna with pedicel plus flagellum 1.2x breadth of head; scape about 3x as long as broad; anellus about as long as broad; funicle virtually filiform but not slender, first funicle segment (F1) hardly narrower than F2, F1 plus F2 as long as pedicel, F1 to F6 all somewhat longer than broad, F7 subquadrate, funicle segments not separated by peduncles, each with a single transverse row of linear sensilla and bristly setae, the setae only about one quarter of segment breadth; clava ovate, bluntly pointed, 2.4x as long as broad, slightly broader than funicle.
Mesoscutum and scutellum with strong reticulate sculpture, dull; scutellum dorsally almost flat. Acropleuron as in female, coriaceous anteriorly, almost smooth posteriorly. Fore wing with ratio lengths of costal cell: marginal vein: stigmal vein: postmarginal vein as 34:24:8:11. Propodeum almost horizontal, callus with setae only behind and laterad of spiracle (abraded?).
Gaster oval, 1.25x as long as mesosoma, 2.15x as long as broad, its dorsal surface with relatively strong, transversely reticulate sculpture.
Before Gibson’s (2010) recognition that the males from El Ventorrillo belonged to C. bolivari, they had been identified by RRA as C. aestivalis. C. bolivari and C. aestivalis are morphologically close and male C. bolivari and male C. aestivalis run out together at couplet 13 in Askew & Nieves-Aldrey’s (2006: 88) key. Males of C. bolivari may be distinguished from those of C. aestivalis by their rather shorter and stouter antennae and more uniform colouration of the mesoscutum; in C. aestivalis there are usually more or less distinct longitudinal submedian bands of purplish colour separated by a median green band, but this colour banding is absent, or at most only vaguely indicated, in C. bolivari. Males of the two species can be further distinguished by sculpture of the frontovertex. Similar to females (Gibson, 2010), males of C. aestivalis have a reticulate sculpture whilst those of C. bolivari have a coriaceous sculpture with obvious piliferous punctures.
Calosota carmenae Askew & Nieves-Aldrey sp. n. † (Figs. 1, 2, 3A-B)
|
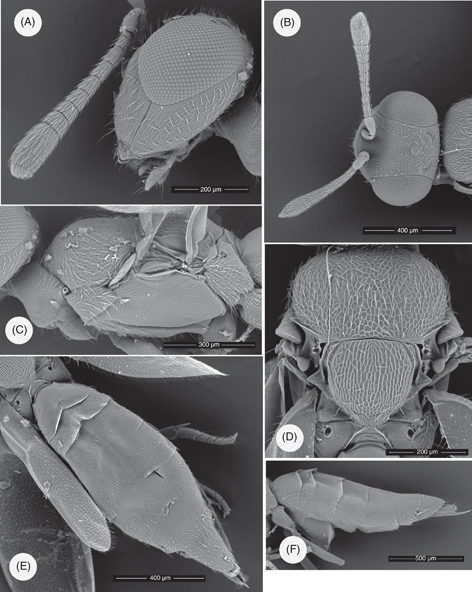 Fig. 1.— Calosota carmenae n. sp., adult female (SEM): (A) Head and antenna, lateral view. (B) Head and antennae, dorsal view. (C) Mesosoma, lateral view. (D) Mesosoma, dorsal view. (E) Metasoma, dorsal view. (F) Metasoma, lateral view. Fig. 1.— Calosota carmenae n. sp., adult female (SEM): (A) Head and antenna, lateral view. (B) Head and antennae, dorsal view. (C) Mesosoma, lateral view. (D) Mesosoma, dorsal view. (E) Metasoma, dorsal view. (F) Metasoma, lateral view.
Fig. 1.— Calosota carmenae n. sp., hembra adulta (SEM): (A) Cabeza y antena izquierda, en visión lateral. (B) Cabeza y antenas en vision dorsal. (C) Mesosoma en vision lateral. (D) Mesosoma en visión dorsal. (E) Metasoma en visión dorsal. (F) Metasoma en visión lateral.
|
|
|
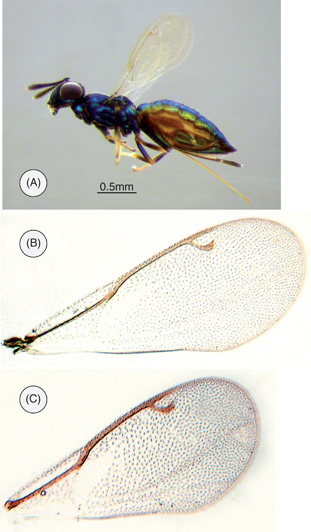 Fig. 2.— Calosota carmenae n. sp.: (A) Habitus, female. (B) Female fore-wing. (C) Male fore-wing. Fig. 2.— Calosota carmenae n. sp.: (A) Habitus, female. (B) Female fore-wing. (C) Male fore-wing.
Fig. 2.— Calosota carmenae n. sp.: (A) Habitus de la hembra. (B) Ala anterior de la hembra. (C) Ala anterior del macho.
|
|
|
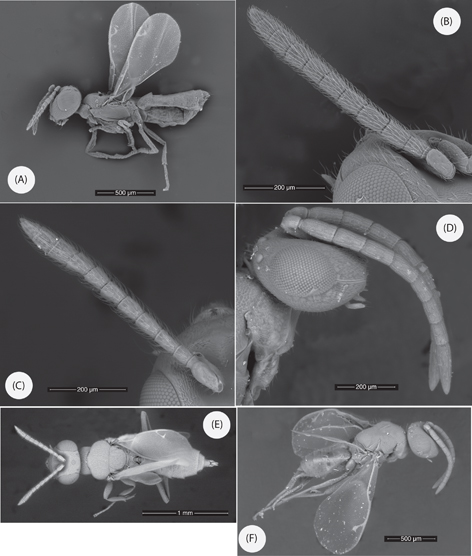 Fig. 3.— Calosota carmenae n. sp.: (A) Habitus, male lateral view. (B) Male antenna. (C) Calosota bolivari Askew, male antenna. (D) Anastatus oscari (Ruthe), male head and antenna, lateral view. (E) Calosota bolivari, habitus male, dorsal view. (F) Anastatus oscari, habitus male lateral view. Fig. 3.— Calosota carmenae n. sp.: (A) Habitus, male lateral view. (B) Male antenna. (C) Calosota bolivari Askew, male antenna. (D) Anastatus oscari (Ruthe), male head and antenna, lateral view. (E) Calosota bolivari, habitus male, dorsal view. (F) Anastatus oscari, habitus male lateral view.
Fig. 3.— Calosota carmenae n. sp.: (A) Habitus del macho en visión lateral. (B) Antena del macho. (C) Calosota bolivari Askew, antena del macho. (D) Anastatus oscari (Ruthe), cabeza y antenas del macho en visión lateral. (E) Calosota bolivari, habitus del macho en visión dorsal. (F) Anastatus oscari, habitus del macho en visión dorsal.
|
|
urn:lsid:zoobank.org:act:76E9568A-8A96-424F-B469-1721BE13E3A2
TYPE MATERIAL. Holotype ♀. SPAIN, Almería, Mojácar, La Parata. 137 m. 37º 07´14.9”N, 1º51´0.6”W. Malaise trap 24.vi -21.vii.2013, C. Rey leg. [in Museo Nacional de Ciencias Naturales, Madrid, Spain (MNCN), card mounted. Cat. no. 2787].
PARATYPES: 6♂♂, 18♀♀, same data as holotype, captured in Malaise trap 5-24.vi.2013, except 3♂♂ captured 6-21.vii. 2♂♂ and 2♀♀ paratypes mounted on stubs, coated with gold, for observation under SEM. In MNCN.
ADDITIONAL MATERIAL. 91 individuals captured in Malaise trap, same data as the type series. All captures of C. carmenae in the La Parata Malaise trap, 103♀♀ and 16♂♂, were distributed over the following collecting periods in 2013: 2–13.v (13♀♀, 2♂♂), 13.v – 24.vi (40♀♀, 3♂♂), 24.vi–21.vii (25♀♀, 4♂♂), 21.vii–3.viii (21♀♀, 4♂♂), 3–28.viii (4♀♀).
FEMALE (Fig. 2A). Length 2.4 mm. Head purple-black, upper face and vertex blue-green with broad violet bands; antennal scape pale yellow with a small apical brown area; clava with oblique apical surface pale yellow; palps black. Pronotum and mesoscutum blue; scutellum blue with extensive purple-black tints; propodeum shining blue-green; prepectus and anterior half or more of acropleuron violet, posteriorly blue-green. Wings clear, setation brownish, venation pale. Femora and tibiae dark brown, protibia with a small pale apical mark, mesotibia and mesofemur with more extensive pale marks apically and ventrally, and metatibia and metafemur with still more extensive pale apical marks; tarsi pale, only pretarsi brown. Gaster green-blue, dorsal surface of T1-T5 mostly violet.
Head in dorsal view (Fig. 1B), about 1.9x as broad as long; eyes separated by 0.42x head breadth; temples about 0.17x head length; POL about 2.1x OOL, OOL slightly greater than diameter of ocellus, ocellar triangle slightly acute; vertex with quite strongly raised reticulate sculpture. Head in front view about 1.2x as broad as high; toruli centred at or slightly below lower eye margin; frons with raised reticulate sculpture, bottoms of antennal scrobes smooth and shiny laterad of weakly sculptured intertorular prominence but with raised reticulation in dorsal halves. Antenna (Figs. 1A, 1B), with pedicel plus flagellum 1.1x as long as head breadth; scape 0.75x height and 0.8x transverse diameter of eye, about 4.5x as long as broad; pedicel twice as long as broad, as long as anellus plus first funicle segment (F1); anellus weakly transverse; F1 about 1.6x as long as but scarcely broader than anellus, narrower than pedicel, F2 to F5 longer than broad, F6 and F7 somewhat transverse, flagellum very gradually widening to F4 and thereafter more strongly to F7, F7 about twice as broad as F1; linear sensilla in a single transverse row on each funicle segment, two visible in lateral view on most segments; setae on funicle segments short and bristly, standing out at no more than 30°; clava in profile about 1.9x as long as broad, its apex an obliquely truncate pale pad of micropilosity.
Mesosoma (Figs. 1C, 1D), 1.7x as long as broad; mesoscutum 1.3x as broad as long with strongly raised reticulate sculpture; scutellum as broad as long, convex in profile, appearing longitudinally striate with sculpture of narrow elongated areoles, anterior margin about 3.3x breadth of an axilla. Acropleuron mostly coriaceous with weakly raised reticulate sculpture in part. Metacoxa with femoral depression shallow, bare. Propodeum medially shorter than dorsellum; spiracle separated from anterior margin by rather more than its major diameter which exceeds median length of propodeum.
Fore wing (Fig. 2B), not quite reaching base of last gastral tergite; basal cell almost bare, basal vein with a few pale setae; cubital vein with sparse, white setae beneath basal cell, strongly upcurved to basal vein; wing beyond basal vein with a distinct speculum reaching below base of marginal vein anteriorly but separated from cubital vein by a broad band of pale setae, disc of wing with short, dense pilosity. Lengths of costal cell: marginal vein: stigmal vein: postmarginal vein as 25:20:6:7; stigmal vein curved, forming angle to postmarginal vein of about 45°.
Gaster (including ovipositor) (Figs. 1E, 1F) 1.7x as long as mesosoma, in dorsal view 2.9x as long as broad; ovipositor sheath exserted beyond apex of T7 by about twice its depth in profile.
MALE. Differing from female mostly in antennal and gastral characters. Antennal scape brown, non-metallic, narrowly yellowish brown basally and ventrally. Scutellum green-blue with few purple tints, not contrasting in colour with mesoscutum. Length 2.2mm, smaller than female.
Antennal scape about 3.0x as long as broad, slightly expanded subapically; pedicel plus flagellum 1.2x as long as breadth of head; flagellum almost filiform; pedicel about as long as anellus plus F1; F1 shorter and narrower than F2 but following funicle segments subequal in length, all longer than broad with setae dense and rather more outstanding than in female; clava about 2.4x as long as broad, as long as last three funicle segments together and only slightly broader than funicle, bluntly pointed.
Fore wing (Fig. 2C) with basal cell bare, setation brownish, speculum developed and extending below base of marginal vein, as in female.
Gaster 1.15x as long as mesosoma, in dorsal view 2.4x as long as broad, long ovate, dorsally flattened; T7 downturned, cercal setae reaching slightly beyond apex.
ETYMOLOGY. Named for Carmen Rey, a good friend and collaborator of the junior author, who collected and sorted the Malaise trap samples from La Parata.
COMMENTS. Calosota carmenae and C. metallica (Gahan) females share the characters of a developed fore wing speculum (the only known Calosota species to have this character), clavate antennal flagellum, relatively elongate gaster and extensive bright green-blue colouration. C. carmenae is readily distinguished from C. metallica by its pale-coloured antennal scape (yellowish in the female, brownish in the male). In C. metallica the scape is dark, usually yellowish at most at its base, and with metallic tints, although Gibson (2010) mentions seven Spanish females collected in 1973 and 1974 which have a mostly yellowish scape that is slightly darkened only apically. Calosota dusmeti Bolívar y Pieltain also has a pale female scape, but the fore wing speculum is absent, the female gaster is only 2.4x as long as broad, the antennal flagellum is not clavate and it is a larger species, about 3 mm in length. Colouration of the weakly sculptured acropleuron also differs consistently in Spanish specimens of C. carmenae and C. metallica being violet over the anterior half or more and green-blue posteriorly in C. carmenae and mostly green-blue with usually only flecks of violet medially and posteriorly in C. metallica.
Calosota dusmeti Bolívar y Pieltain, 1929
Calosota dusmeti and C. obscura Ruschka are similar, differing in particular in the paler scape and legs of the former, and it is possible that they are colour variants of a single species (Askew & Nieves-Aldrey, 2006). Should it be eventually shown that C. dusmeti is distinct from C. obscura, then it might be known under the name C. violascens (Masi, 1922) (Gibson, 2010). Female material with partly yellowish scapes is mentioned from Portugal by Gibson (2010).
A female in MNCN collected at Rivas Vaciamadrid, 20.viii.2002 J.I. López-Colón, listed in Askew & Nieves-Aldrey (2006), has been labelled by Lucian Fusu in 2012 as ‘cf violascens’.
Calosota metallica (Gahan, 1922)
Calosota viridis Masi, 1922
Calosota matritensis Bolívar y Pieltain, 1929
Calosota coerulea Nikol’skaya, 1952
Gibson (2010) synonymised C. viridis, under which name the species is discussed by Askew & Nieves-Aldrey (2006), with C. metallica from western Canada and the USA. Calosota metallica is known in North America as a primary parasitoid of Mayetiola destructor (Say) and other Cecidomyiidae in wheat and stems of other Poaceae, and of species of Tetramesa (Hymenoptera, Eurytomidae) in similar situations. It is also known as a facultative secondary parasitoid. In North America C. metallica appears to be primarily parthenogenetic with males very scarce, but in Europe males are quite plentiful (Gibson, 2010).
Specimens (in BMNH) were reared by Ribes from stems of Stipa parviflora with Tetramesa collected in 2007 in Lleida (Montoliu).
Calosota modesta Bolívar y Pieltain, 1929 †
Calosota modesta was synonymised under C. viridis Masi (= C. metallica) by Askew & Nieves-Aldrey (2006) after examination of the male holotype, but removed from this synonymy by Gibson (2010) who considers the male holotype and one of the female paratypes of C. matritensis Bolívar y Pieltain (= C. metallica) to be conspecific. Further study is required to establish the status of C. modesta.
Calosota nitens Askew, 2006
Described from 3♀♀ collected by a Malaise trap in Madrid (El Pardo) in 1991 (Askew & Nieves-Aldrey, 2006), an additional female was swept in Lleida (Sarroca), 13.vii.2011 (RRA) and a male and female were captured in a Malaise trap in Almería (Mojácar), 21.vii - 3.viii.2013 (CR). The male is described below. Gibson (2010) provides an additional distinguishing character of the female; there are long setae along the propodeal petiolar foramen in addition to those on the callus. The females from Mojácar have very setose propodeal calli and several setae behind spiracles up to the posterior margin of the propodeum. The male is much less setose with only two or three setae immediately posterior to a spiracle.
MALE. Antennal scape brown, non-metallic, the rest of antenna a slightly darker brown. Head and mesosoma coloured much as in female; metasoma with three basal gastral tergites brown, non-metallic. Length 1.5mm.
Differs from female principally in antennal and gastral characters. Antennal scape about 3x as long as broad, only slightly longer than half the height of an eye; pedicel plus flagellum 1.25x as long as width of head; pedicel in profile about 1.5x as long as deep; anellus transverse, anellus plus F1 shorter and narrower than pedicel; F2 as broad as pedicel, F3 to F7 slightly longer than broad, progressively widening so that F7 is about 1.3x as broad as F3; clava as long as three preceding funicle segments, and C1 and C2 1.3x as broad as F7 (but somewhat flattened in drying), C3 narrow, only as broad as F3; setae on funicle segments standing out at about 40°, curved, forming a fringe that on middle segments is fully half as wide as breadth of segments.
Metacoxa with some setae in femoral depression, but not as conspicuously pilose as in female. Forewing ratio lengths of costal cell: marginal vein: stigmal vein: postmarginal vein as 18:15:5:6.
Gaster a little shorter than head plus mesosoma (40.5:42), in dorsal view 2.6x as long as broad.
Calosota obscura Ruschka, 1921
Calosota obscura is frequently reared from the stems of herbaceous plants which contain the galls of cynipids of the tribes Aulacideini, Aylacini and Phanacidini (Hym., Cynipidae) (Askew & Nieves-Aldrey, 2006), although the actual host of the eupelmid has not been ascertained. New records of C. obscura associated with cynipid galls are of 1♂ emerging from stems of Cichorium intybus containing galls of Timaspis cichorii (Kieffer), Lleida, Santa Engracia near Tremp, 2009, RRA, and of 1♂ reared from galls of Iraella luteipes (Thomson) in stems of Papaver somniferum, Madrid, Valdemorillo, 2004, JLNA. There is 1♀ in MNCN, determined by Gibson and by RRA, labelled as emerging from stems of Polygala comosa (Polygalaceae), Cádiz, Aguilillas, 2004, JLNA.
There are five females from Madrid Province (El Pardo, Montarco, Vaciamadrid) in MNCN under C. obscura, and identified as such by RRA, bearing labels ‘Calosota ?violascens Masi det. G. Gibson 2010’ and also ‘Calosota obscura Ruschka det. Fusu, L. 2012’.
Calosota lixobia Erdös, 1946 was proposed as a synonym of C. obscura (Askew & Nieves-Aldrey, 2006) but Gibson (2010) suggests instead possible synonymy under C. violascens Masi, 1922 (see also above under C. dusmeti). A male from Madrid, El Pardo in MNCN, collected by G. Mercet, has been determined as C. lixobia by Gibson in 2010 and as C. obscura by Fusu in 2012.
Calosota vernalis Curtis, 1836
One female from Malaise trap, Almería (Mojácar, 24.vi-21.vii.2013, CR) (MNCN).
Eusandalum Ratzeburg, 1852
No information is available on the species of Eusandalum found in Iberia and the Canary Islands additional to that in Askew & Nieves-Aldrey (2006).
Eusandalum coronatum (Thomson, 1876)
Eusandalum flavipenne Ruschka, 1921
Eusandalum ibericum (Bolívar y Pieltain, 1923
Eusandalum inerme (Ratzeburg, 1848)
Eusandalum merceti (Bolívar y Pieltain, 1926)
Eupelmus seyrigi (Bolívar y Pieltain, 1926)
Eusandalum walkeri (Curtis, 1836)
Pentacladia Westwood, 1835
Pentacladia elegans Westwood, 1835
Additional to the locations cited for P. elegans in Askew & Nieves-Aldrey (2006), it was found by AR at sites in Lleida (Aitona, Montoliu, Sarroca) (BMNH) and both sexes were collected by CR in Almería (Mojácar) (MNCN). The host remains unknown.
NEANASTATINAETOP
Metapelma
Metapelma nobile (Förster, 1860)
No new data
Neanastatus Girault, 1913
Neanastatus africanus Ferrière, 1938 † (Fig. 4D)
|
 Fig. 4.— Female habitus of some selected Iberian species of Eupelmidae: (A) Anastatus giraudi (Ruschka). (B) Eupelmus (Macroneura) aseculatus Kalina. (C) Eupelmus (Episolindelia) linearis Förster. (D) Neanastatus africanus Ferrière. (E) Eupelmus (Macroneura) muellneri Ruschka. (F) Eupelmus (Episolindelia) pallicornis Gijswijt. Fig. 4.— Female habitus of some selected Iberian species of Eupelmidae: (A) Anastatus giraudi (Ruschka). (B) Eupelmus (Macroneura) aseculatus Kalina. (C) Eupelmus (Episolindelia) linearis Förster. (D) Neanastatus africanus Ferrière. (E) Eupelmus (Macroneura) muellneri Ruschka. (F) Eupelmus (Episolindelia) pallicornis Gijswijt.
Fig. 4.— Habitus de la hembra de especies ibéricas seleccionadas de Eupelmidae: (A) Anastatus giraudi (Ruschka). (B) Eupelmus (Macroneura) aseculatus Kalina. (C) Eupelmus (Episolindelia) linearis Förster. (D) Neanastatus africanus Ferrière. (E) Eupelmus (Macroneura) muellneri Ruschka. (F) Eupelmus (Episolindelia) pallicornis Gijswijt.
|
|
Here newly recorded from Spain (and Europe) on the basis of many Malaise trapped specimens from Almería, Mojácar, La Parata (leg. CR): 5♀♀, 3♂♂ 2-13.v.2013, 5♀♀, 8♂♂ 13.v-24.vi.2013, 13♀♀, 1♂ 24.vi-21.vii.2013, 7♀♀ 21.vii-3.viii.2013, 2♀♀, 3-28.viii.2013 (MNCN).
Neanastatus africanus was described from south-west Africa. Ferrière (1938) reports the rearing of a specimen from the gall of an Asphondylia species on Sesamum (Sim-sim) in Uganda.
Neanastatus turneri Ferrière, 1938
A single female was Malaise trapped, together with 11♀♀ N. africanus, in Almería (Mojácar, 24.vi-21.vii.2013, CR) (MNCN).
EUPELMINAETOP
Anastatus Motschulsky, 1859
Anastatus bernardi Ferrière, 1954
No information additional to that in Askew & Nieves-Aldrey (2004).
Anastatus bifasciatus (Geoffroy in Fourcroy, 1785)
Found at Mont-rebei (Lleida) by AR (pers. comm.).
Anastatus catalonicus Bolívar y Pieltain, 1935
The publication date of the description of A. catalonicus was erroneously given by Askew & Nieves-Aldrey (2004) as 1934.
Anastatus giraudi (Ruschka, 1921) (Fig. 4A)
One female collected Madrid (Rivas-Vaciamadrid, área de Montarco, 10.vii.2004, J.I. Lopez-Colón), and another female (with three males) in Almería (Mojácar, La Parata, Malaise trap 13.v - 24.vi.2013, CR) (MNCN). The latter female has wings reaching to the apex of the gaster (Fig. 4A); all of our records from Spain and the Canary Islands are of the macropterous form. A female in MNCN (catalogue number 84551) is from Tenerife, Bajamar, 6.vi.1935 (no other data).
Anastatus japonicus Ashmead, 1904
No information additional to that in Askew & Nieves-Aldrey (2004).
Anastatus lichtensteini (Ruschka, 1921)
No information additional to that in Askew & Nieves-Aldrey (2004).
Anastatus maculosus Askew, 2004
No information additional to that in Askew & Nieves-Aldrey (2004).
Anastatus magnoculus Askew, 2004
Anastatus magnoculus was described from a single female from Tenerife. Two additional females have recently been found in MNCN, both with the same data: Tenerife, Médano, 13-25.xii.1932. No collector is given on the label but the handwriting is very neat and probably that of A. Cabrera.
Anastatus oscari (Ruthe, 1859)
Both sexes of A. oscari were collected in Carmen Rey’s Malaise trap sample from La Parata, Mojácar, Almería (2♂♂ 2-13.v.2013, 6♂♂ 13.v-24.vi.2013, 1♀, 3♂♂ 24.vi-21.vii.2013, 2♀♀, 6♂♂ 21.vii-3.viii.2013, 1♀ 3-28.viii.2013) (MNCN). Recognition of the male resulted in the identification of 4♂♂ collected by J. Blasco-Zumeta in Zaragoza (Pina de Ebro) (RRA collection): 1♂ 20.vi.1991 Malaise trap, 2♂♂ 18.ix.1990 Malaise trap, 1♂ ex Stephaniola salsolae (Tavares) (Dipt. Cecidomyiidae) galls on Salsola vermiculata collected 22.viii.1991 (S. salsolae is unlikely to be the actual host; species of Anastatus are mainly parasitoids in eggs of orthopteroid hosts, less frequently in eggs of Hemiptera, Lepidoptera or other orders). A brief description of the hitherto unrecognised male follows.
MALE. Body green to bluish green but mesepisternum violet. Antenna uniform brown, the scape with weak metallic green reflections. Legs dark except about femoral-tibial joints, anterior face of protibia, meso- and metatibial spurs and basal three or four tarsomeres of middle and hind legs. Wings clear, venation light brown. Length 1.3-1.6 mm.
Head in dorsal view 1.95x as broad as long; POL 2.4x OOL, OOL 1.6x ocellar diameter. Head in front view rounded, slightly broader than high (25:23), minimum eye separation 0.6x head breadth, antennal toruli just above level of lower eye margin, face below toruli with relatively stout, downwardly-directed, white setae. Antenna with scape only slightly expanded apically and, excluding radicle, just over 3x as long as broad; pedicel plus flagellum not quite 2x head breadth, pedicel 2x as long as broad, anellus transverse; first funicle segment (F1) about 2.4x as long and 1.3x as broad as pedicel, its ventral surface very weakly concave, about 2.7x as long as broad, F2 to F7 progressively slightly shorter and narrower than preceding antennomere, F7 about 2.2x as long as broad; clava about as long as F6+F7; all funicle segments with extremely short pilosity and short linear sensilla arranged in irregular transverse rows, 3 rows on F1 to F5, 2 on F6 and F7.
Mesosoma 1.4x as long as broad, mesoscutum with quite strong reticulate sculpture, more shiny than head but less shiny than scutellum, the latter with only weakly raised reticulation. Fore wing fully developed, lengths of costal cell: marginal vein: stigmal vein: postmarginal vein as 120:63:28:36. Propodeum half as long as scutellum, median area almost smooth, median carina complete but weak.
Gaster ovate, 0.9x as long as mesosoma, 1.6x as long as broad, tergites with quite strong sculpturation and comparatively conspicuous white setae.
The male of A. oscari will run to couplet 17 in the key to species of Anastatus from Spain and the Canary Islands in Askew & Nieves-Aldrey (2004), but it is distinguishable from the three species therein by having a dark, relatively elongated scape that is over three times as long as broad. The metatibia is virtually entirely dark as in A. bernardi.
Anastatus uromeni Ferrière, 1968 †
2♂♂, 2♀♀ Huesca (Fraga) 31T BF58, ex Ferula communis stems, collected 15.iii.2010, emerged 2010, AR (BMNH).
Our identification of this species is based upon the description (Ferrière, 1968) of the original material of five females reared from eggs of Uromenus brevicollis insularis Chopard (Ephippigeridae) in stems of Asphodelus ramosus from Sardinia (Italy). The male (described below) was unknown to Ferrière. There is good general agreement between the two Spanish females and the original description, the only discrepancies being that the Spanish specimens have rather less greenish colouration on predominantly black bodies with violet tints, relatively shorter ovipositor sheath about 0.6x length of gaster and not quite 0.8x length of metatibia (respectively 0.75x and 1.0x in the description), and the reduced fore wing reaches to about the apex of the fourth gastral tergite, well beyond the middle of the gaster. Differences between the Spanish specimens and the original description of A. uromeni are considered insufficient to support the belief that two species are involved. It is probable that the host of the Spanish specimens in stems of F. communis (Apiaceae, Giant Fennel) was an orthopteran.
MALE. Body green, head bluish green with a few violet tints in the ocellar region; gaster with base and apex green but dorsal surface otherwise coppery. Antenna with scape yellow, dorso-apically metallic green. Palps yellow. Legs with femora metallic green except for yellow trochanters and about apical one-quarters of pro- and mesofemora; protibia yellow with brown streak on flexor surface, mesotibia with brown subapical mark covering one-third of dorsal surface, metatibia dark brown with only basal one-third and apex (narrowly) yellow. Wings clear, venation brown. Length 2.3mm.
Head in dorsal view 1.7x as broad as long, POL 2.6x OOL, OOL 1.7x ocellar diameter. Head in front view rounded, 1.15x as broad as high, minimum eye separation 0.5x head breadth, antennal toruli slightly above level of lower margin of eye, face below toruli with relatively short and fine downwardly-directed whitish setae. Antenna with scape expanded apically, excluding radical about 2.1x as long as broad; anellus transverse; F1 with ventral surface concave, 2.7x as long as broad and about 1.5x as broad as pedicel; F2 as broad as F1 and 2.3x as long as broad, the following funicle segments scarcely narrower than F1 but progressively shortening; F7 about 1.5x as long as broad; clava slightly longer than F6+F7 (14:12) with a flat ventral surface; all funicle segments with very short linear sensilla arranged in 5 or 6 irregular transverse rows on F1 to F5, thereafter in 3 or 4 rows, and with extremely short pilosity.
Mesosoma 1.5x as long as broad, mesonotum moderately shiny with irregular raised sculpture, only slightly weaker on scutellum than on mesoscutum, notauli rather deep. Fore wing fully developed, lengths costal cell: marginal vein: stigmal vein: postmarginal vein as 33:17:8:12; stigma quite large, triangular, separated from postmarginal vein by its apical depth. Propodeum medially about half length of scutellum, median area shiny with weak sculpture which is strongest adjacent to the complete median carina.
Gaster ovate, about as long as mesosoma, dorsally with slightly raised reticulate sculpture, rather dull with dark setae.
The male of A. uromeri runs in the key in Askew & Nieves-Aldrey (2004) as far as couplet 16 (although the scutellar base occupies rather more than one-third of the distance between the posterior ends of the notauli). The dark band on the metatibia extends over more than half the length of the tibia as in A. catalonicus, but the head is slightly less than 1.7x as long as A. giraudi. The mesotibial dark band is longer and darker in A. uromeni than in A. giraudi.
Arachnophaga Ashmead, 1896
Arachnophaga matritensis (Bolívar y Pieltain, 1934)
The description of A. matritensis (in Mercetina) was published in 1934 and not 1933.
Arachnophaga picardi (Bernard, 1936)
No additional information to that in Askew & Nieves-Aldrey (2004).
Brasema Cameron, 1884
Brasema stenus (Bouček, 1968)
Brasema ephedricola Askew, 1998
Several specimens of both sexes were reared from stems of Cichorium intybus containing galls of Timaspis cichorii (Hym., Cynipidae), the probable host and a new record, Lleida (Sta Engracia near Tremp, RRA and AR). From stems collected 1.v.2009 Brasema emerged vii.2009, later than other parasitoid species (RRA collection), and those collected 23.iii.2010 produced Brasema vi.2010 (AR).
1♂ swept Juniperus phoenicea, Lleida (Aitona, 2.iii.2007, AR).
2♀♀ were Malaise trapped, Almería (Mojácar, La Parata, vi & vii.2013, CR) (MNCN).
Brasema sp. indet.
Four specimens of Brasema in MNCN, as yet unidentified but not B. stenus and possibly representing two species, were collected on Tenerife (Canary Islands) by A. Cabrera:
1♀, Santa Cruz, Bajamar, 15.x.1903, MNCN Ent 84555 (body green, tegulae yellow, legs yellow, except coxae metallic, pro- and metafemora with small brown marks and last tarsal segments dark).
1♀, Bca Hondo, 23.x.1904 (apex of gaster and ovipositor sheath missing)
1♀, Laguna, 7.v.1910
1♀, Santa Cruz, Bajamar, 10.xi.1908, MNCN Ent 137133
Calymmochilus Masi, 1919
Calymmochilus delphinus Askew, 2004
Recorded from the Canary Islands (La Palma) by Fusu, Askew & Ribes (in press). A number of specimens were found in the Malaise trap samples collected in Almería (Mojácar, La Parata, 2013, CR): 2♀♀ 2-13.v, 1♀ 13.v-24.vi, 2♀♀ 24.vi-21.vii, 2♂♂ 21.vii-3.viii (MNCN and RRA collections).
Calymmochilus dispar Bouček & Andriescu, 1967
Additional records for this species are from Cuenca (El Cubillo, 1.x.2005, J.I. López-Colón) (MNCN) and Lleida (Mont-rebei. pre-2008, AR) (BMNH).
Calymmochilus russoi Gibson, 1995 †
A full account of the discovery of this species by AR in Lleida is recorded in Fusu, Askew & Ribes (in press). It was reared from galls of Parapodia sinaica (Frauenfeld) (Lep., Gelechiidae) found on Tamarix canariensis at Torres de Segre.
Calymmochilus subnubilus (Walker, 1872)
No records additional to those in Askew & Nieves-Aldrey (2004).
Eupelmus Dalman, 1820
Eupelmus (Episolindelia) australiensis (Girault, 1913) †
A cosmopolitan species, added to the Spanish faunal list by Ribes (2011). It attacks Cecidomyiidae (Dipt.) in Poaceae and is well known as a parasitoid of the sorghum midge, Stenodiplosis sorghicola (Coquillet). Ribes’ specimens, now in BMNH and RRA collections, were obtained in Lleida (Torres de Segre, vii and x.2010) by sweeping Sorghum halepense.
Eupelmus australiensis is also present in the Canary Islands (new record), La Gomera, above Santiago, 28.iii.1999, 1♀ (RRA) and La Gomera, Playa del Ingles in Valle Gran Rey, 27.iii.1999, 1♀ (RRA)
Eupelmus (Episolindelia) clavicornis Askew, 2000
Gibson & Fusu (2016) place E. clavicornis in the subgenus Episolindelia. The species was described from specimens reared from galls of Etsuhoa thuriferae Skuhravá (Cecidomyiidae) on Juniperus thurifera in Zaragoza. One female has subsequently been found in Segovia (Pedraza, 29.vi.2007, RRA) and another female was reared from the same host galls and plant from Lozoya (Madrid, 27.ii.2017, JLNA leg) (MNCN).
Eupelmus (Episolindelia) fuscipennis Förster, 1860
To the few records of this species in Askew & Nieves-Aldrey (2000, 2004), we can add four Malaise trapped specimens: 1♀ Madrid, El Ventorrillo, 11.vii.1991, A. Garrido; 1♀ Madrid, El Pardo, viii.1991, JLNA & CR; 2♀♀ Zaragoza, Pina de Ebro, 6.vii.1991 J. Blasco-Zumeta (MNCN and RRA collections). The species has been redescribed by Vikberg (2008) from material reared from an egg batch of Cicadetta montana (Scopoli) (Homoptera) in Finland, and a male and female emerged from a stem of Centaurea nigra collected in France (Lot et Garonne, 1994, RRA).
Eupelmus (Episolindelia) juniperinus Bolívar y Pieltain, 1934
The date of publication of E. juniperinus is 1934, not 1933 as misquoted in Askew & Nieves-Aldrey (2000). In this paper it was noted as being associated with Juniperus oxycedrus (as the typical subspecies) and with J. thurifera (as subspecies thuriferae Askew). In an unpublished document dated 2008, Antoni Ribes records rearing both sexes of E. juniperinus from galls of ‘Oligotrophus sp.’ (probably Etsuhoa (Cecidomyiidae)) on J. phoenicea (Lleida, San Llorenç de Montgai, 2007). A female from this sample in RRA’s collection has a relatively short ovipositor sheath, 0.33x as long as the metatibia, much as in subspecies thuriferae, but it differs in colouration from the latter in being considerably darker with yellowish markings on the mesosoma limited to the posterior margin of each mesoscutal ridge, metanotum and medial part of the propodeum. The side-lobe of the mesoscutum is without an isolated metallic green oval spot, present in thuriferae, and the metallic areas are dark green with coppery to violet tints medially, not bright green as in thuriferae.
Eupelmus (Episolindelia) linearis Förster, 1860 (Fig. 4C)
Seemingly uncommon in Spain, an unpublished list of chalcids found in Lleida by A. Ribes, dated 2008, includes this species on the basis of specimens reared from stems of Festuca paniculata subsp. spadicea growing at Ager. Additionally, 2♀♀ were Malaise trapped in Almería (Mojácar, La Parata, 2-13.v.2013, CR) (MNCN).
Eupelmus (Episolindelia) moroderi Bolívar y Pieltain, 1934
The date of publication of E.moroderi is 1934, not 1933 as misquoted in Askew & Nieves-Aldrey (2000).
Eupelmus (Episolindelia) pallicornis Gijswijt, 1993 (Fig. 4F)
This species was placed in subgenus Episolindiella by Gibson & Fusu (2016). It is a parasitoid in galls of Etsuhoa (Cecidomyiidae) on Juniperus and was known only from Spain (Soria, Zaragoza). The species has now been now found also in Madrid, Lozoya, reared from the same host, galls of Etsuhoa thuriferae on Juniperus thurifera, collected 27.ii.2017: 1♂, 1♀ emerged iii.2017 (JLNA) (MNCN).
Eupelmus (Episolindelia) testaceiventris (Motschulsky, 1863)
Abundant in the Canary Islands, the only record additional to those given in Askew & Nieves-Aldrey (2000) is a female, identified by A. Ribes, collected on Euphorbia balsamifera, La Gomera (Alajeró, 6.ii.2010, K. Trøjelsgaard).
Eupelmus (Eupelmus) acinellus Askew, 2009 †
Eupelmus acinellus was described from many specimens of both sexes reared from fruits of Juniperus phoenicea collected in Lleida (Juncosa, Sant Llorenç de Montgai) (2007 and 2009, A. Ribes), Zaragoza (Pina de Ebro) (1992, J. Blasco-Zumeta) and the Canary Islands, La Gomera (La Culata) (1999, RRA, mistakenly identified as E. urozonus in Askew & Nieves-Aldrey, 2000). The host in Lleida was Mesophleps oxycedrella (Millière) (Lep., Gelechiidae), established by finding live E. acinellus larvae beside the larval remains of the gelechiid in dissected J. phoenicea fruits. 1♂, 1♀ E. acinellus also emerged from fruits of J. oxycedrus, collected at Juncosa on 26.xii.2007 and emerging 23.v.2008 (A. Ribes) (RRA collection). E. acinellus appears to be univoltine, emerging in summer from once-overwintered Juniperus fruits and adults have been swept from J. phoenicea in late June (Ribes Escolà & Askew 2009).
Eupelmus (Eupelmus) annulatus Nees, 1834
Records in Askew & Nieves-Aldrey (2000) under this name, presented prior to the revision of the ‘Eupelmus urozonus complex’ by Al khatib et al. (2014, 2015), refer to Eupelmus azureus Ratzeburg (below). E. annulatus is, however, a Spanish species (Gibson & Fusu, 2016). Among some Eupelmus collected by A. Ribes and examined by G. Gibson, there is a female E. annulatus, determined by Gibson in 2015, which was reared 6.vii.2008 from a fruit of Pistacia lentiscus collected 20.x.2007 at S. Llorenç Montgai (Lleida). A male which we identify as E. annulatus was reared from Lymantria dispar (L.) (Lep., Lymantriidae), probably from a cocoon, in Menorca (Alaior, vii.2010, J. Pujade-Villar) (RRA collection).
Eupelmus (Eupelmus) atropurpureus Dalman, 1820
Found commonly in Lleida (Aitona, Algerri, Fulleda, La Granja d’Escarp, Montoliu, Mont-rebei, Sarroca, Torres de Segre, Tremp, Utxesa) and also recorded from Huesca (Fraga), E. atropurpureus was swept from Euphorbia serrata, Atriplex halimus, Lygos sphaerocarpa, Suaeda vera and mixed vegetation from April to early July and again, more commonly, in September and October (A. Ribes) (BMNH). Specimens were reared from galls of Lasioptera eryngii (Vallot) (Dipt., Cecidomyiidae) on Eryngium campestre, Isocolus lichtensteini (Mayr) and Timaspis cichorii (Hym., Cynipidae) on Centaurea aspera and Cichorium intybus respectively, and from the larval cases of a species of Coleophora (Lep., Coleophoridae) on Suaeda vera; also from unknown hosts in Artemisia herba-alba fruits, Atriplex halimus stems and fruits, Dactylis glomerata stems, Dorycnium hirsutum fruits, D. pentaphyllum fruits, Helichrysum stoechas flower heads, Hordeum vulgare stems, Scorzonera angustifolia heads, Stipa parviflora stems, from Suaeda vera, and apparently from Coccoidea (Hem.) on Ephedra distachya although this last record requires confirmation (A. Ribes, RRA).
Elsewhere in Spain, E. atropurpureus was reared as a secondary parasitoid of Euphydryas aurinia (Rottemburg) (Lep., Nymphalidae) via Cotesia melitaearum (Wilkinson) (Hym., Braconidae) cocoons and of Euphydryas desfontainii (Godart) via Cotesia sp. cocoons, and also as a presumed pseudohyperparasitoid from a Euphydryas sp. pupa (Barcelona, 2003, C. Stefanescu) (RRA collection). 1♀ emerged from galls of Phanacis hypochoeridis (Kieffer) (Hym., Cynipidae) (Madrid, Mt San Pedro, 2006, RRA). E. atropurpureus females were found in the field in Madrid (Montarco, 31.viii.2003, J.I. López Colón; Ordel, C. Bolívar y Pieltain), Navarra (Sos del Rey Católico, 12.vi.2006, RRA) and Salamanca (Ciudad Rodrigo) (MNCN).
Eupelmus atropurpureus has been identified from Portugal (Gibson & Fusu, 2016).
Eupelmus (Eupelmus) azureus Ratzeburg, 1844 †
Eupelmus spongipartus Förster, 1860
Prior to the recognition of additional species in the ‘E. urozonus complex’ (Al khatib et al., 2015), specimens with a relatively long ovipositor sheath (about as long as the marginal vein) were identified as E. annulatus and those with a shorter sheath as E. urozonus. Eupelmus azureus, however, has a long sheath like E. annulatus; the two species are readily separable on the sculpturation of the scrobal depression which is mainly smooth and shiny in E. azureus but largely reticulate in E. annulatus (Al khatib et al., 2015; Gibson & Fusu, 2016). Eupelmus annulatus has a broad range of hosts but E. azureus is most frequently reared from galls of Cynipidae on Quercus. In Spain E. azureus has been found as a parasitoid in galls of Andricus coriarius (Hartig) asex. gen. (Madrid, El Escorial on Q. pyrenaica, 1983, JLNA; Salamanca, Villanueva del Conde, 1978, JLNA), A.hispanicus (Hartig) asex. gen. (Salamanca, Linares de Riofrío, 1978, JLNA ; Madrid, Alameda del Valle, 1987, JLNA; Lleida, Os de Balaguer on Q. faginea, 2008, A. Ribes ; Picos de Europa, Brez, 2013, RRA), A. legitimus Wiebes-Rijks asex. gen. (Salamanca, Membribe de la Sierra, 1980, JLNA), A. grossulariae (Wachtl) asex. gen. (Salamanca, Tenebrón, 1978, JLNA), A. pictus (Hartig) asex. gen. (Segovia, Revenga on Q. petraea, 2017, JLNA), A. quercusramuli (L.) sex. gen. (Lleida, Granadella, Menàrguens and Els Torms, 2006 and 2008, AR), A. quercustozae (Bosc) asex. gen. (Ciudad Real, Fuencaliente on Q. faginea, 1984, JLNA; Toledo, Buenasbodas- Robledo on Q. faginea, 1989, JLNA), A. solitarius (Boyer de Fonscolombe) asex. gen. (Salamanca, La Alberca, 1978, JLNA), Biorhiza pallida (Olivier) sex. gen. (Lleida, Les Avellanes, Menàrguens and Pobla de Cérvoles on Q. faginea and Q. petraea, 2006-2012, AR), Neuroterus quercusbaccarum (L.) (Lleida, Menàrguens, 2007, AR) and Plagiotrochus australis (Mayr) sex. gen. (Madrid, Guadalix de la Sierra, 2015, RRA). In addition, E. azureus has been reared from galls of Dryocosmus kuriphilus Yasumatsu on Castanea sativa (Cantabria, 2014, S. Gutiérrez) (JLNA material mostly in MNCN, AR material mostly in BMNH).
Eupelmus azureus is listed from Portugal by Gibson & Fusu (2016).
Eupelmus (Eupelmus) cerris Förster, 1860
New record: 2♂♂ reared from galls of Synophrus hispanicus Hartig (Hym., Cynipidae) on Quercus suber, Cáceres, Plasencia, collected x.1986, emerged iii.1987, JLNA (MNCN).
Eupelmus (Eupelmus) confusus Al khatib, 2015 †
Spanish and Canary Islands material of this recently distinguished species in the ‘Eupelmus urozonus complex’ has been reared from fruits of Asphodelus ramosus with a Bruchophagus sp. (Hym., Eurytomidae) (3♀♀, 1♂, Mallorca, Sóller, 2011, J. Biblioni), from fruits of Juniperus phoenicea together with Eupelmus acinellus (above) (1♀, Canary Islands, La Gomera, La Culata, 1999, RRA), fruits of Olea europaea with Batrocera oleae (Rossi) (Dipt. Tephritidae) (1♀, Tarragona, La Conia, 1967, J. Templado; 1♂ Málaga, Marbella, 1967, J.A. Fiestas, with E. martelli at both sites), galls of Myopites stylatus (Fabricius) (Dipt. Tephritidae) on Inula (1♀, 2♂♂, Tarragona, La Selva del Camp, 2011, AR), galls of Amblypalpis sp. (Lep., Gelechiidae) on Tamarix canariensis (1♀, Lleida, Torres de Segre, 2012 and 2♀♀, Huesca, Fraga, 2011, AR), and from a gall of Diplolepis rosae (L.) (Hym., Cynipidae) together with Eupelmus urozonus (below) (1♀, Madrid, Guadalix de la Sierra, 2014, RRA). One male was swept from Ephedra fragilis, 4.vi.2008, La Granja d’Escarp (Lleida), AR. Gibson & Fusu (2016) identified E. confusus from Mallorca.
In the Canary Islands it was reared from fruits of Juniperus phoenicea containing Lepidoptera larvae (La Gomera, Vallehermosa, 1999, RRA), and taken in a Malaise trap on Tenerife (Las Cañadas, 1994, P. Oromi) (RRA collection).
Eupelmus (Eupelmus) gemellus Al khatib, 2015 †
This is the species associated with Ephedra in the Monegros region of Zaragoza, recorded under ‘Eupelmus sp.’ as a parasitoid of Blascoa ephedrae Askew (Hym., Pteromalidae) in the seeds (Askew & Blasco-Zumeta, 1997) and as a primary and secondary parasitoid of the gall-forming Eurytoma gallephedrae Askew (Askew & Blasco-Zumeta, 1998).
Specimens of E. gemellus reared by A. Ribes, their identity confirmed by G. Gibson, are as follows:
2♀♀ from galls of Eurytoma gallephedrae Askew (Hym., Eurytomidae) on Ephedra nebrodensis, Huesca (Fraga), 2009; 4♀♀, 1♂ from seeds of Ephedra distachya, Lleida (Montoliu), 2009; 5♀♀, 2♂♂ from seeds of Ephedra fragilis, Lleida (La Granja d’Escarp), 2008; 1♀ from seed of Ephedra nebrodensis, Huesca (Fraga), 2010; 1♀ from fruit of Juniperus phoenicea, Lleida (Llorenç Montgai), 2009; 1♀ from fruit of Pistacia lentiscus, Lleida (Llorenç, Montgai), 2007 (BMNH).
Additionally 2♂♂, 6♀♀ were reared from unidentified eurytomid galls on roots of Ephedra nebrodensis. Madrid, Monte Pajares, 21.v.2004, JLNA leg. (MNCN).
Eupelmus gemellus was captured in the field, by A. Ribes in Lleida, sweeping Pinus halepensis (1♂ 5.v.2005, Torrebeses), Ephedra fragilis (6♀♀, 3♂♂ 4.vi.2008, La Granja d’Escarp), Juniperus oxycedrus (1♀ 5.v.2005, Torrebeses) and J. phoenicea (1♀ 27.vi.2009, Juncosa).
Eupelmus gemellus is also reported from Portugal (Gibson & Fusu, 2016: 118).
Eupelmus (Eupelmus) iranicus Kalina, 1988 † & E. (E.) longicorpus Girault, 1915
A single female in RRA’s collection, labelled ‘Jaen, S. Spain, 20.vii.1974, R.R. Askew’ is tentatively identified as E. iranicus using the key in Gibson & Fusu (2016). It is broken with the head (minus antennae), mesosoma, one fore wing, the legs of one side only and gaster all mounted separately on a card. The previously known distribution of E. iranicus is Israel, Libya, Somalia and Greece (Kos).
Morphologically similar species to E. iranicus are E. kalinai Gibson & Fusu, 2016 (= algiricus Kalina, 1988) and E. longicorpus Girault, 1915. Gibson & Fusu (2016: 127) suggest that E. iranicus and/or E. kalinai could be junior synonyms of E. longicorpus, but they exclude E. longicorpus from their list of Palaearctic Eupelmus. Eupelmus kalinai and E. longicorpus are parasitoids of Cecidomyiidae in grasses.
Eupelmus longicorpus, the first chalcid name in Australasian Chalcidoidea (Bouček, 1988: 2), is given as an example of a species with a very broad distribution. It was described from Australia and has been found in India, Zimbabwe and Spain, the only Spanish record apparently being Bouček’s (1988: 561).
Eupelmus kalinai Gibson & Fusu, 2016 †
Eupelmus kalinai is a replacement name for E. algiricus Kalina, 1988 which is a junior homonym of E. (Macroneura) algiricus (Kalina, 1981) (Gibson & Fusu, 2016). It is reported from two Spanish localities by Gibson & Fusu (2016) on the basis of museum material: Castellón, Benicasim, 1974, BMNH and Tarragona, south-west of Salou, 1952, RMNH).
Eupelmus (Eupelmus) kiefferi De Stefani, 1898 †
Two female specimens of E. kiefferi from Spain (Segovia and Zaragoza) were misidentified in Askew & Nieves-Aldrey (2000) as E. fulvipes Förster (Gibson & Fusu, 2016: 142). Eupelmus fulvipes should be deleted from the Spanish list.
Specimens of E. kiefferi, identified by G. Gibson, were collected in Lleida by A. Ribes only at La Mitjana and Torres de Segre (BMNH and RRA collections). At the former locality 1♂ was reared from a seed-head of Althaea officinalis in 2006 and 2♀♀ were swept from this plant on 29.iii and 24.vii.2006, 1♀ was reared from a gall of Amblypalpis (Lep., Gelechiidae) on Tamarix canariensis in 2012 and 1♀ was swept from this plant on 29.viii.2006, 2♂♂ were reared from seed-heads of Lythrum salicaria in 2012, and 1♀, 1♂, originally identified as E. fulvipes, emerged from galls of Diplolepis rosae (Hym., Cynipidae) in 2006. At Torres de Segre 2♀♀ were swept from mixed vegetation on 5.x.2012. In addition, material now recognised as E. kiefferi, formerly reported as E. urozonus (Askew & Nieves-Aldrey, 2000), was reared from galls of Myopites (Dipt., Tephritidae) on Inula (1♀ Girona, Tossa, 1961, RRA and 1♂ Majorca, 1982, M. Boness). Also, from galls of Diastrophus rubi (Cynipidae) on Rubus (3♀♀, 3♂♂ Lugo, Souto de S. Miguel, 14.iii.2017, JLNA & Tapetado leg. and from a gall of Diplolepis rosae (1♀ Aragón, Jaca, 1992, RRA).
Eupelmus (Eupelmus) longicalvus Al khatib & Fusu, 2015 †
Gibson & Fusu (2016: 159) record a specimen from Ávila, Peguerinos, obtained by sweeping Pinus sylvestris, 26.viii.1994, JLNA.
Eupelmus (Eupelmus) martellii Masi, 1941 †
In MNCN there are specimens reared by J.A. Fiestas from Bactrocera oleae (Rossi) (Dipt., Tephritidae): 10♀♀ and 2♂♂, Tarragona, La Cenia, 1967 and 12♀♀, 1♂, Málaga, Marbella, 1967. Another female and male from these series we identify as E. confusus. A female which we believe to be E. martellii was swept 13.vii.1994 in Zaragoza (Nuévalos, JLNA). The report by Ruschka (1921) of ‘Eupelmus urozonus’ from Olea near Barcelona may refer to E. martellii.
Eupelmus (Eupelmus) matranus Erdös, 1947
Eupelmus splendens Bolívar y Pieltain, 1933 (homonym of E. splendens Giraud, 1871)
Eupelmus matranus is seldom found in Spain, being reported in Askew & Nieves-Aldrey (2000, 2004) only from the type of its synonym E. splendens Bolívar y Pieltain, 1933 nec Giraud, 1871, and from a Mercet specimen in MNCN. Additional records are of 3♀♀ from Lleida in A. Ribes’ collection (now in BMNH), two swept from Quercus ilex on 21.v.2005 at Granadella and one from Q. faginea on 8.vi.2008 at Ciutadilla. A female in MNCN was reared from a gall of Andricus burgundus Giraud sex. gen. on Q. suber (Girona, Roses, 1988, JLNA).
Eupelmus (Eupelmus) memnonius Dalman, 1820 †
Gibson & Fusu (2016: 182) record this species from Spain (material in RMNH).
Eupelmus (Eupelmus) microzonus Förster, 1860
A frequent parasitoid in galls on herbaceous plants, particularly those formed by Cynipidae (Hym.) and Tephritidae (Dipt.), the following records of reared specimens, additional to those in our previous papers, have been obtained from:
Galls of Cynipidae: Aulacidea freesei Nieves-Aldrey on Silybum marianum (Madrid, Arganda, 2002, RRA); Aulacidea laurae Nieves-Aldrey on Podospermum laciniatum (Madrid, Vaciamadrid, 2004, JNLA); Aulacidea tragopogonis (Thomson) on Tragopogon (Madrid, Arganda and Vaciamadrid, 2002, RRA); Aylax minor Hartig in Papaver rhoeas seed capsules (Madrid, Arganda, 2003 and Vaciamadrid, 1989, JLNA; Lleida, Tremp, 2003, RRA); Barbotinia oraniensis (Barbotin) in Papaver rhoeas seed capsules (Catalunya, Cardona, 2003, RRA; Guadalajara, Valdenoches, JLNA); Isocolus lichtensteini (Mayr) on Centaurea sp. (Madrid, Arganda, 2003, RRA); Diplolepis mayri (Schlechtendal) (Toledo, 2013, RRA) and Diplolepis rosae on Rosa (Lleida, Utxesa, 2010, AR).
Galls of Diptera: unidentified host cecidomyiids on Medicago sativa (Jerez, 2009, I. Sánchez) and on Atriplex (Lleida, Torres de Segre, 2010, RRA). Galls of Myopites sp. (Tephritidae) (Madrid, Aranjuez, 2004, RRA) produced E. urozonus, as did those of Urophora sp. on Centaurea collected in Portugal (Algarve, Ferragudo, 1979, M. Boness), and a specimen emerged with an unidentified tephritid from coalesced achenes of Tragopogon (Soria, Hortezuela, 2007, RRA).
Other hosts: a primary or secondary parasitoid of Blascoa ephedrae Askew (Hym., Pteromalidae) in seeds of Ephedra fragilis (Zaragoza, Sierra de Alcubierre, 1997, J. Blasco-Zumeta) and of Eurytoma gallephedrae Askew {Hym., Eurytomidae) in galls on Ephedra nebrodensis (Madrid, Carabaña, 1995, S. Arce-Castilla); unknown host in stem of Onopordum (Toledo, 2004, RRA); unknown host in seed-head of Salvia lavandulifolia (Madrid, Arganda, 2003, RRA).
Additional specimens of E. microzonus were reared by A. Ribes from Centaurea aspera flower-heads (Lleida, Serós, 2008, identified by G. Gibson), Helichrysum stoechas flower-heads (Lleida), Papaver rhoeas seed capsules (Lleida) and by sweeping at Fraga (Huesca, 26.vi.2009), Juncosa (Lleida, 27.vi.2009) and Sarroca (Lleida), 16.v.2009).
New distributional records: Spain, Navarra, Sos del Rey Católico (swept), 2006, RRA; Almería, Mojácar, La Parata, 2013, CR; Canary Islands, Tenerife, Pinar de la Esperanza, 1928 (MNCN). The occurrence of E. microzonus in Mallorca is cited by Gibson & Fusu (2016: 186).
Eupelmus (Eupelmus) nitidus Nikol’skaya, 1952 †
Reported from Spain by Gibson & Fusu (2016: 196), a male and female, identified as E. nitidus by L. Fusu, emerged in 2012 from galls of Amblypalpis sp. (Lep., Gelechiidae) collected from Tamarix canariensis, Lleida (Torres de Segre, AR).
Eupelmus (Eupelmus) opacus Delvare, 2015 †
This recently described species was recorded from Spain by Gibson & Fusu (2016: 199). 1♀, 2♂♂ emerged from fruits of Betula pendula from Lleida (Les), collected by A. Ribes, viii.2008, the males overwintering to 2009 but the female emerging 29.x.2008 (specimens identified by G. Gibson). A species of Semudobia (Dipt., Cecidomyiidae) is a probable host; Gibson & Fusu (2016) also report the rearing of E. opacus from Rabdophaga salicis (Schrank) galls on Salix in Ukraine (M. Zerova).
? Eupelmus (Eupelmus) phragmitis Erdös, 1955 †
A male, identified as E. phragmitis by G. Gibson and now in BMNH, was reared by A. Ribes 10.vii.2011 from a stem of Heteropogon contortus collected i.2011 in Tarragona (La Selva del Camp). The identity of the specimen is queried because it is not mentioned by Gibson & Fusu (2016) who indicate a possibly exclusive association of E. phragmitis with Phragmites and do not report it from the Iberian Peninsula.
Eupelmus (Eupelmus) pini Taylor, 1927
Askew & Nieves-Aldrey (2000) recorded this species from Andorra under the name E. aloysii Russo (synonymy Gibson, 2011). No additional material has been located.
Eupelmus (Eupelmus) prionini Delvare, 2015 †
This is another species in the urozonus-group. It is reported from Portugal (1♀, Beira Alta, Valhelhas, 1999, M.J. Gijswijt) by Gibson & Fusu (2016: 230).
Eupelmus (Eupelmus) splendens Giraud, 1871
Eupelmus splendens may be restricted to attacking hosts in galls of Pediaspis aceris (Gmelin) (Hym., Cynipidae). Spanish specimens were recorded from galls collected in Catalonia (Pujade-Villar, 1989), and in 2007 and 2008 A. Ribes reared both sexes from galls on Acer monspessulanum from two localities in Lleida (Pobla de Cérvoles, Mont-rebei).
Eupelmus (Eupelmus) stenozonus Askew, 2000
The type material of E. stenozonus is from La Gomera and Tenerife, Canary Islands. Additional male specimens, identified by G. Gibson, were collected by K. Trøjelsgaard in 2010 from flowers of Launaea arborescens (La Gomera, Alajerö) and Rubus fruticosus (Tenerife, Fosnia). In MNCN several specimens were found that had probably been collected by Cabrera in Tenerife (1♀, Tejina, 1903; 7♀♀, Bajamar, 1904; 3♀♀, El Mediano, 1923). Gibson & Fusu (2016: 261) add Libya to the range of this species and indicate Capitites ramulosa and Myopites species (Dipt., Tephritidae) on Asteraceae as hosts.
Eupelmus (Eupelmus) stramineipes Nikol’skaya, 1952 †
An addition to the Spanish list based upon a female swept by A. Ribes in Lleida (Utxesa, 17.vii.2008) and identified by G. Gibson, 2015 (now in BMNH).
Eupelmus (Eupelmus) urozonus Dalman, 1820
Portugal is included in the distribution of E. urozonus by Gibson & Fusu (2016: 294).
Several specimens of E. urozonus, their identity confirmed by G. Gibson in 2015, were reared by A. Ribes in Lleida from oak galls of Cynipidae (Hym.): Andricus hispanicus asex. gen. on Quercus faginea, Mont-rebei, 2008; Biorhiza pallida sex. gen, on Q. faginea, Mont-rebei, 2008, on Q. petraea, Les, 2008 and 2009, on Q. robur, Les, 2009 and Cynips disticha Hartig asex. gen. on Q. faginea, Les Avellanes, 2008. AR also reared the species from galls of Mikiola fagi (Hartig) (Dipt., Cecidomyiidae) on Fagus sylcatica, Pantà de Senet, 2008 and Artiga de Lin, 2012.
Other reared material of E. urozonus, specific identity confirmed, has been examined: ex Andricus coriarius (Hartig) asex. gen. on Quercus pyrenaica (Madrid, El Escorial, JLNA); A. hispanicus asex. gen. and A. quercustozae asex. gen. on Quercus (Picos de Europa, Brez, 2012, RRA); A. quercusradicis (Fabricius) sex. gen. (Salamanca, Monsagro, JLNA); A. quercustozae asex. gen. on Quercus faginea (Toledo, Buenasbodas and Castilla-La Mancha, Pozo de Guadalajara, JLNA); Dryocosmus kuriphilus on Castanea sativa (Galicia, labelled ‘Orense, Concello de Amoeiro, 2014, S. Perez Otero); Plagiotrochus australis on Q. ilex (Madrid, Colmenar Viejo, 2014, RRA); P. quercusilicis (Fabricius) sex. gen. (Madrid, El Escorial, JLNA); Diplolepis mayri and D. rosae on Rosa (Madrid, Guadalix de la Sierra, 2014, RRA).
The association of E. urozonus with Etsuhoa thuriferae (Cecidomyiidae) on Juniperus thurifera (in Soria) (Gijswijt, 1993) is confirmed, with rearings from Zaragoza, Pina de Ebro (1997, J. Blasco-Zumeta).
Material identified as E. urozonus, that was reared from galls of Eurytoma gallephedrae Askew (Eurytomidae) on Ephedra nebrodensis in Askew & Blasco-Zumeta (1998), is mostly correctly determined, although some E. confusus (above) were also obtained from this host (Zaragoza, Monegros, J. Blasco-Zumeta). Also, specimens recorded as E. urozonus that were reared from fruits of Juniperus phoenicea, together with E. acinellus (Ribes Escolà & Askew 2009), collected in both Zaragoza and La Gomera, are now identified as E. confusus.The probable host in J. phoenicea fruits is Mesophleps oxycedrella (Millière) (Lep., Gelechiidae).
1♀ was swept from Q. petraea, Lleida (Bossòst) on 8.viii. 2006, RRA.
Eupelmus (Macroneura) aseculatus Kalina, 1981 (Fig. 4B)
Another record of E. aseculatus as a parasitoid in acorn galls of Callirhytis glandium (Giraud) (Cynipidae): 1♀ Salamanca, Teso Santo, 1982, JLNA. Malaise trap samples from Almería (Mojácar, La Parata, 2013, CR) contained 3♀♀ (13.v-24.vi), 9♀♀ (24.vi-21.vii), 8♀♀ (21.vii-3.viii) and 5♀♀ (3-28.viii) (MNCN and RRA collections).
Eupelmus (Macroneura) falcatus (Nikol’skaya, 1952)
A female was swept in Lleida (Sarroco,13.vii.2011, AR) (RRA collection) and two males, determined by G. Gibson, were collected in Lleida, one reared from a stem of Cichorium intybus (Tremp, 2010, AR) (BMNH) and one swept (Aitona, 30.vii.2011, AR) (RRA collection).
Eupelmus (Macroneura) maculatus (Ferrière, 1954)
Our only additional record is of 1♀ collected in a Malaise trap in Almería (Mojácar, La Parata, 2-13.v.2013, CR) (MNCN).
Eupelmus (Macroneura) muellneri Ruschka, 1921 (Fig. 4E)
In addition to the records in Askew & Nieves-Aldrey (2000), where E. muellneri was first reported from mainland Spain, specimens were reared from cecidomyiid galls on Artemisia (Murcia, Fortuna, M.J. Gijswijt, 1992) and by AR in Lleida from Lasioptera eryngii (Cecidomyiidae) galls on Eryngium campestre, Lepidoptera galls on Tamarix canariensis and inflorescences of Atriplex halimus. 10♀♀ and 95 probable ♂♂ were collected in a Malaise trap in Almería (Mojácar, La Parata, 2013, CR). A female in MNCN emerged from a gall of Plagiotrochus quercusilicis sex. gen. (Cynipidae) (Madrid, Robledo de Chavela, 1986, JLNA).
Eupelmus (Macroneura) seculatus (Ferrière, 1954)
Reared from galls of Stefaniola salsolae (Tavares) and Stefaniola sp. (Cecidomyiidae) on Salsola vermiculata in Lleida (Aitona, Aspa, 2008, AR), and obtained in a Malaise trap in Almería (Mojácar, La Parata, 2013, CR). In the latter sample it was less common than E. (M.) aseculatus (above) with only 5♀♀ collected compared to 39♀♀ E. (M.) aseculatus.
The male is described by Pujade-Villar (1989).
‘Eupelmus (Macroneura) vesicularis (Retzius, 1783) aggregate’
It is now recognized that an aggregate of species is confused under this name (Fusu, 2010, 2017) and at least two of these species are represented in the Spanish fauna. An account of the Iberian species that were hitherto cited as Eupelmus (Macroneura) vesicularis will be presented after the publication of Fusu’s (2017) revision.
A parasitoid of endophytic insects, particularly of those in herbaceous plants, ‘E. (M.) vesicularis agg.’ was found in abundance in Lleida and Huesca by AR who reared specimens from galls of Lasioptera eryngii (Cecidomyiidae) on Eryngium campestre and Timaspis cichorii (Cynipidae) on Cichorium intybus, from stems of Brachypodium retusum and Stipes parviflora and from fruits of Astragalus stella and Medicago sativa.
Specimens of the dark ‘form’ (Fusu, 2010) in MNCN were reared by JLNA from cynipid oak galls of Andricus quercusradicis sex. gen. (Salamanca, Cuidad Rodrigo, 1979), Callirhytis glandium asex. gen. (Salamanca, Tres Santo, 1982), Cynips divisa Hartig asex. gen. (Salamanca, Peñaparda,1978; Cáceres, Madrigal de la Vera, 1989, Quercus pyrenaica), Neuroterus albipes (Schenck) sex. gen. (Salamanca, La Honfría, 1979), Trigonaspis bruneicornis Kieffer asex. gen. (Salamanca, Peñahorcada, 1978) and T. mendesi Tavares asex. gen. (Segovia, Siguero, Q. faginea).
Merostenus Walker, 1837
Merostenus excavatus (Dalman, 1820)
No further data additional to that in Askew & Nieves-Aldrey (2004) are available.
The four species which follow were formerly placed in the genus Reikosiella Yoshimoto, 1969, but in a recent publication Gibson (2017) treats Reikosiella as a synonym of Merostenus.
Merostenus andriescu Fusu, 2013 †
The female holotype in MNCN (Ent 82435), the sole known specimen, was collected in the Canary Islands (Tenerife, Monte de Tagana) on 15.v.1922 (Fusu, 2013).
Merostenus bolivari (Kalina, 1988) †
Described from Algeria, this species was recorded from Spain by Fusu (2013: 9) on the basis of a female reared by A. Ribes from a gall of Plagiotrochus australis (Mayr) (Hym., Cynipidae) from Lleida (Algeri, 2007). Fusu (2013) suggests that two females Malaise trapped in Madrid (El Pardo, 1-8.ix.1991), queried as Eupelmus rostratus by Askew & Nieves-Aldrey (2004: 37), may be M. bolivari and we are able to here confirm that this is indeed the case.
Merostenus hungarica (Erdös, 1959)
Three Spanish females (from Madrid, Jaén and Zaragoza) are listed by Askew & Nieves-Aldrey (2000, 2004) and another, found in Valencia (Saler) in 1989, is noted in Fusu (2013: 19). A further two females were captured in a Malaise trap in Almería (Mojácar, La Parata, v-vi.2013, CR).
Merostenus rostrata (Ruschka, 1921)
Askew & Nieves-Aldrey (2000, 2004) previously mention this species, as Eupelmus rostratus, from Zaragoza. Specimens from Madrid discussed under this name belong to M. bolivari (see above).
Tineobius Ashmead
Tineobius tamaricis Ribes & Fusu, 2017 †
A species recently described from Spanish material and belonging to a genus previously unknown from the Palaearctic region, T. tamaricis was reared by A. Ribes from galls of Parapodia sinaica (Frauenfeld) (Lep., Gelechiidae) collected from Tamarix canariensis. A total of seven females emerged from or were found dead inside galls collected at Torres de Segre, Pantá de Camelis, 31T BG80 and BF99 (Lleida) x.2007, ii.2010, i.2011, i.2012, x.2012, i.2013, emergences in the summer following gall collection, the only available exact dates being 22.viii.2011 from gall collected 8.i.2011. Large numbers of galls were collected and T. tamaricis is clearly a rare parasitoid (in comparison, 186 specimens of a microgasterine Braconidae were reared from the samples). That T. tamaricis is a parasitoid of P. sinaica is suggested by the finding of a gelechiid larval head capsule in a gall beside an unemerged T. tamaricis (Fusu & Ribes, 2017), but secondary parasitism must be a possibility.
AcknowledgementsTOP
We are very grateful for records received from the late Antoni Ribes, and for the cooperation of Maria Theresa Escolà in allowing us access to his collection. Information and suggestions supplied by Gary Gibson and Lucian Fusu, both in their published work and in personal communications, have been invaluable in improving this paper.The considerable contribution made by Carmen Rey’s Malaise trap samples from Almería is gratefully acknowledged. Material kindly made available by P. Oromi was collected during the project ‘Inventory of the invertebrate fauna in Teide National Park’ sponsored by Organismo Autónomo de Parques Nacionales. Financial support of RRA at the Museo Nacional de Ciencias Naturales (CSIC) in Madrid was provided by the European Community’s Programme ‘Research Infrastructure Action’ under SYNTHESYS (ES-TAF-4868). JLNA was supported in part by research projects (MINECO/FEDER, UE) CGL2015-66571-P, (AEI/FEDER, UE) AGL2016-76262-R and Encomienda de Gestión del MAPAMA a la Agencia Estatal CSIC, exp. 16MNES003.
ReferencesTOP
| ○ |
Al khatib, F., Fusu, L., Cruaud, A., Gibson, G., Borowiec, N., Rasplus, J.-Y., Ris, N. & Delvare, G., 2014. An integrative approach to species discrimination in the Eupelmus urozonus complex (Hymenoptera, Eupelmidae), with the description of 11 new species from the Western Palaearctic. Systematic Entomology, 39: 806–862. http://dx.doi.org/10.1111/syen.12089 |
| ○ |
Al khatib, F., Fusu, L., Cruand, A., Gibson, G., Borowiec, N., Rasplus, J.-Y, Ris, N. & Delvare, G., 2015. Availability of eleven species names of Eupelmus (Hymenoptera, Eupelmidae) proposed in Al khatib et al. (2014). ZooKeys, 505: 137–145. http://dx.doi.org/10.3897/zookeys.505.9021 |
| ○ |
Askew, R. R. & Blasco-Zumeta, J., 1997. Parasitic Hymenoptera inhabiting seeds of Ephedra nebrodensis in Spain, with descriptions of a phytophagous pteromalid and four other new species of Chalcidoidea. Journal of Natural History, 31: 965–982. http://dx.doi.org/10.1080/00222939700770471 |
| ○ |
Askew, R. R. & Blasco-Zumeta, J., 1998. Insects associated with galls of a new species of Eurytomidae (Hymenoptera: Chalcidoidea) on Ephedra nebrodensis in Spain. Journal of Natural History, 32: 805–821. http://dx.doi.org/10.1080/00222939800770431 |
| ○ |
Askew, R. R. & Nieves-Aldrey, J. L., 2000. The genus Eupelmus Dalman, 1820 (Hymenoptera, Chalcidoidea, Eupelmidae) in peninsular Spain and the Canary Islands, with taxonomic notes and descriptions of new species. Graellsia, 56: 49–61. http://dx.doi.org/10.3989/graellsia.2000.v56.i0.309 |
| ○ |
Askew, R. R. & Nieves-Aldrey, J. L., 2004. Further observations on Eupelminae (Hymenoptera, Chalcidoidea, Eupelmidae) in the Iberian Peninsula and Canary Islands, including descriptions of new species. Graellsia, 60: 27–39. http://dx.doi.org/10.3989/graellsia.2004.v60.i1.191 |
| ○ |
Askew, R. R. & Nieves-Aldrey, J. L., 2006. Calosotinae and Neanastatinae in the Iberian Peninsula and Canary Islands, with descriptions of new species and a supplementary note on Brasema Cameron, 1884 (Hymenoptera, Chalcidoidea, Eupelmidae). Graellsia, 62: 87–100. http://dx.doi.org/10.3989/graellsia.2006.v62.i1.28 |
| ○ |
Bolívar y Pieltain, C., 1934. Estudio de algunos Eupélmidos nuevos de España (Hym. Chalc.). Eos, 9: 195–209. |
| ○ |
Bolívar y Pieltain, C., 1935. Estudio monográfico de las species españolas del género Anastatus Motsch. (Hym. Chalc.). Eos, 10: 273–292. |
| ○ |
Bouček, Z., 1988. Australasian Chalcidoidea (Hymenoptera): a biosystematic revision of genera of fourteen families, with a reclassification of species. C.A.B. International. Wallingford. 832 pp. |
| ○ |
Ferrière, C., 1938. Eupelmides exotiques [Hymenopt. Chalcididae] I. Les genres Metapelma Westw., Anastoidea Gahan et Neanastatus Girault. Annales de la Société Entomologique de France, 107: 25–72. |
| ○ |
Ferrière, C., 1968. Notes sur quelques chalcidiens nouveaux ou peu connus (Hym.). Mitteilungen der Schweizerischen Entomologischen Gesellschaft, 40: 240–248. |
| ○ |
Fusu, L., 2010. Species status of two colour morphs of Eupelmus vesicularis (Hymenoptera: Eupelmidae) as revealed by allozyme electrophoresis, morphometric and host preference data. Journal of Natural History, 44: 1113–1129. http://dx.doi.org/10.1080/00222931003632773 |
| ○ |
Fusu, L., 2013. A revision of the Palaearctic species of Reikosiella (Hirticauda) (Hymenoptera, Eupelmidae). Zootaxa, 3636: 1–34. http://dx.doi.org/10.11646/zootaxa.3636.1.1 |
| ○ |
Fusu, L., 2017. An integrative taxonomic study of European Eupelmus (Macroneura) (Hymenoptera: Chalcidoidea: Eupelmidae), with a molecular and cytogenetic analysis of Eupelmus (Macroneura) vesicularis: several species hiding under one name for 240 years. Zoological Journal of the Linnean Society, 181(3): 519–603. https://doi.org/10.1093/zoolinnean/zlw021 |
| ○ |
Fusu, L., Askew, R. R. & Ribes, A., in press. Rediscovery of Calymmochilus russoi Gibson, 1995 (Hym., Chalcidoidea, Eupelmidae), new to Spain and another host record. Zootaxa. |
| ○ |
Fusu, L. & Ribes, A., 2017. Description of the first Palaearctic species of Tineobius Ashmead, 1896 with DNA data, a checklist of world species, and nomenclatural changes in Eupelmidae (Hymenoptera, Chalcidoidea). European Journal of Taxonomy, 263: 1–19. http://dx.doi.org/10.5852/ejt.2017.263 |
| ○ |
Gibson, G. A. P., 2010. Calosota Curtis (Hymenoptera, Chalcidoidea, Eupelmidae) – review of the New World and European fauna including revision of species from the West Indies and Central and North America. ZooKeys, 15: 1–75. http://dx.doi.org/10.3897/zookeys.55.490 |
| ○ |
Gibson, G. A. P., 2011. The species of Eupelmus (Eupelmus) Dalman and Eupelmus (Episolindelia) Girault (Hymenoptera: Eupelmidae) in North America north of Mexico. Zootaxa, 2951: 1–97. |
| ○ |
Gibson, G. A. P., 2017. Synonymy of Reikosiella Yoshimoto under Merostenus Walker (Hymenoptera: Chalcidoidea: Eupelmidae) with a checklist of world species and a revision of those species with brachypterous females. Zootaxa, 4255: 1–65. http://dx.doi.org/10.11646/zootaxa.4255.1.1 |
| ○ |
Gibson, G. A. P. & Fusu, L., 2016. Revision of the Palaearctic species of Eupelmus (Eupelmus) Dalman (Hymenoptera: Chalcidoidea: Eupelmidae). Zootaxa, 4081: 1–331. http://dx.doi.org/10.11646/zootaxa.4081.1.1 |
| ○ |
Gijswijt, M. J., 1993. Species of Eupelmus (Hymenoptera: Chalcidoidea) on Spanish juniper. Entomologische Berichten, 53: 10–12. |
| ○ |
Masi, L., 1922. Materiali per una fauna dell’Arcipelago Toscano. XII. Calcididi del Giglio. Terza serie: Eupelminae (seguito), Pteromalinae (partim). Annali del Museo Civico di Storia Naturale Giacomo Doria. Genova, 50: 140–174. |
| ○ |
Pujade-Villar, J., 1989. Primeros datos sobre los eupélmidos asociados a agallas en Cataluñya (Hym., Chalcidoidea, Eupelmidae) con la descripción del macho de Macroneura seculata (Ferrière, 1954). Orsis, 4: 151–160. |
| ○ |
Ribes, A., 2011. Some Chalcidoidea (Hymenoptera) from Lleida new to the Spanish fauna. Boletín de la Sociedad Entomológica Aragonesa, 48: 337–343. |
| ○ |
Ribes Escolà, A. & Askew, R. R., 2009. Chalcidoidea (Hymenoptera) reared from fruits of Juniperus phoenicea, with descriptions of three new species. Boletín de la Sociedad Entomológica Aragonesa, 45: 109–121. |
| ○ |
Ruschka, F., 1921. Chalcididenstudien I. Verhandlungen der Zoologisch-Botanischen Gesellschaft in Wien, 70: 234–315. |
| ○ |
Vikberg, V., 2008. Description of Finnish specimens of Eupelmus (Episolindelia) fuscipennis Förster reared from eggs of Cicadetta montana (Scopoli). Sahlbergia, 14: 60–67. |
Fig. 1.— Calosota carmenae n. sp., adult female (SEM): (A) Head and antenna, lateral view. (B) Head and antennae, dorsal view. (C) Mesosoma, lateral view. (D) Mesosoma, dorsal view. (E) Metasoma, dorsal view. (F) Metasoma, lateral view.