FIRST RECORD OF THE OAK GALL WASP GENUS NEUROTERUS HARTIG, 1840 (HYMENOPTERA, CYNIPIDAE, CYNIPINI) FROM CENTRAL AMERICA WITH DESCRIPTION OF THREE NEW SPECIES FROM PANAMA AND COSTA RICA
E. Medianero1 & J. L. Nieves-Aldrey2
1Programa Centroamericano de Maestría en Entomología / Departamento de Ciencias Ambientales, Universidad de Panamá, C. P. 0824. ORCID ID: http://orcid.org//0000-0002-8430-9034. E-mail: enrique.medianero@up.ac.pa
2Museo Nacional de Ciencias Naturales (CSIC), Departamento de Biodiversidad y Biología Evolutiva, C/ José Gutiérrez Abascal 2, ES-28006 Madrid, Spain. ORCID ID: http://orcid.org//0000-0002-4711-7455. E-mail: aldrey@mncn.csic.es
(corresponding author)
| |
ABSTRACT
Three new species of Neuroterus Hartig, 1840 (Hymenoptera: Cynipidae: Cynipini) are described from Panama and Costa Rica: Neuroterus elvisi sp. n., Neuroterus pulchrigalla sp. n., and Neuroterus glandiphilus sp. n. The new species are the first of the genus Neuroterus recorded from Central America and the Neotropical region. The new species induce galls on Quercus bumelioides Liebm. (Fagaceae, sect. Quercus, White Oaks). Additional evidence of the presence of other unidentified species of Neuroterus in the sampled area is presented. Diagnostic morphological characters, gall descriptions, distributions, host plant and other biological data of the new species are given and discussed.
http://urn:lsid:zoobank.org:pub:48D0C1E1-1D0C-40D8-B890-FFC85AE7A213
Key words: Cynipidae;
Cynipini;
Neuroterus;
oak gall wasps;
Quercus;
Costa Rica;
Panama.
|
| |
RESUMEN
Primera cita del género Neuroterus Hartig (Hymenoptera, Cynipidae, Cynipini) para América Central, con descripción de tres especies nuevas de Panamá y Costa Rica.
Se describen tres nuevas especies del género Neuroterus Hartig, 1840 (Hymenoptera: Cynipidae: Cynipini) de Panamá y Costa Rica: Neuroterus elvisi sp. n., Neuroterus pulchrigalla sp. n. y Neuroterus glandiphilus sp. n. Las nuevas especies representan el primer registro del género Neuroterus para América Central y la región neotropical. Se presenta también evidencia adicional de la presencia de otras especies de Neuroterus no identificadas. Las nuevas especies inducen agallas en Quercus bumelioides Liebm. (Fagaceae, sect. Quercus, robles blancos). Se aportan caracteres diagnósticos, descripciones de las agallas, datos de su distribución, de las plantas hospedadoras y otros datos de biología de las nuevas especies.
Palabras clave: Cynipidae;
Cynipini;
Neuroterus;
avispas de las agallas;
Quercus;
Costa Rica;
Panamá.
|
IntroductionTOP
Species in the family Cynipidae, one of the two largest families included in the Cynipoidea (Insecta: Hymenoptera), are biologically
peculiar because all their representatives are associated with plant galls. They either induce the galls themselves or live
inside galls caused by other insects, most frequently other cynipids but also Chalcidoidea and Lepidoptera (Nieves-Aldrey, 2001; Van Noort et al., 2007; Nieves-Aldrey & San Blas, 2015; Ronquist et al., 2015). According to Ronquist et al. (2015), the family is currently divided into 12 tribes: Aylacini, Aulacideini, Ceroptresini, Cynipini, Diastrophini, Diplolepidini,
Eschatocerini, Paraulacini, Pediaspidini, Phanacidini, Qwaqwaini and Synergini. Of these, Cynipini is the most species-rich
and diverse tribe, with approximately 1,000 species of so-called “oak gall wasps,” cynipids associated with oaks (Quercus species) and other plants of the Fagaceae family (Csóka et al., 2005; Stone et al., 2009).
The vast majority of Cynipini species described have been from the Holarctic region, but more recently, rich faunas of cynipids
are being discovered from the Oriental and the Neotropical regions, historically poorly sampled with regard to cynipids (Liljeblad et al., 2008; Nieves-Aldrey et al., 2009; Stone et al., 2009; Melika et al., 2010; Medianero & Nieves-Aldrey, 2011; Tang et al., 2016). In the Neotropical region, an increasing sampling effort in countries such as Costa Rica, Panama and Colombia has yielded
rich Cynipini fauna, for the most part undescribed. These studies have extended the geographical distribution of genera of
gall wasp such as Amphibolips Reinhard, Disholcaspis Dalla Torre & Kieffer, Loxaulus Mayr, Odontocynips Kieffer, Bassettia Ashmead, Diastrophus Hartig, Andricus Hartig, Callirhytis Förster and Melikaiella Pujade-Villar (Pujade-Villar, 2008; Medianero & Nieves-Aldrey, 2011; Nieves-Aldrey et al., 2013; Medianero & Nieves-Aldrey, 2014; Pujade-Villar & Rodríguez, 2015). At the same time, new genera endemic to this region have been described, for example Agastoroxenia Nieves-Aldrey & Medianero, Coffeikokkos Pujade-Villar & Melika, Barucynips Medianero & Nieves-Aldrey and Zapatella Pujade-Villar & Melika (Nieves-Aldrey & Medianero, 2010; Pujade-Villar et al., 2012a, 2012b; Medianero & Nieves-Aldrey, 2013).
Neuroterus Hartig is one of the more species-rich Cynipini genera along with Andricus Hartig. Since Hartig (1840) described the genus for the first time, based only on European species, Neuroterus has become one of the more problematic Cynipini genera regarding their generic limits and the identification and classifications
of their included species. The reasons for this are diverse. Neuroterus is a taxonomically complex genus, very rich in species and widely distributed around the world; the species are extremely
uniform in morphology, and finding reliable diagnostic characters that allow for their identification is extremely difficult.
Some morphological and molecular phylogenetic studies have found evidence that the genus is not monophyletic with regard to
certain lineages or species groups (Liljeblad et al., 2008; Stone et al., 2009; Melika et al., 2010), as traditionally conceived by Hartig and subsequently by Kinsey (1923). As a consequence, and based on host Quercus associations, some authors have noted that the classification should split some lineages or groups of species closely related
to Neuroterus into the separate genera Pseudoneuroterus Kinsey and Cerroneuroterus Melika & Pujade-Villar. More recently, a new genus closely related to Neuroterus, Cycloneuroterus Melika & Tang, was described for a group of Oriental species associated with the ancestral Fagaceae —Quercus subgenus Cyclobalanopsis, Lithocarpus and Castanopsis— in Taiwan and mainland China (Tang et al., 2011, 2016).
Currently, Neuroterus, as conceived by Melika et al. (2010), includes approximately eighty species from the Holarctic Region. From the United States and Canada, 56 species have been
listed (Kinsey, 1923; Burks, 1979; Melika & Abrahamson, 1997, 2002). Kinsey (1938) recorded six new species from Mexico, and three more have been recently described (Pujade-Villar et al., 2015, 2016), for a total of nine Neuroterus species recorded from that country. The presence of Neuroterus species south of Mexico has been not recorded to date.
In the framework of a continued study of the oak gall wasps (Cynipidae) in the Neotropical region, this paper includes the
description of three new species of Neuroterus from Panama and Costa Rica; this represents the first accurate report of this genus in Central America and the Neotropical
Region.
Material and methodsTOP
STUDY MATERIAL
The adults studied were reared from galls collected from Quercus bumelioides Liebm. and Quercus lancifolia Schledl & Cham., in montane tropical forests in Panama. The Panama samples were collected from December 2007 to May 2009
and November 2016 in several sites in Chiriqui Province, Panama. Additionally, some localities were sampled by the second
author in January 2014 in Costa Rica, where galls from Q. bumelioides yielded one of the new species described. The adult insects emerged from the galls in rearing cages under laboratory conditions.
Voucher specimens of adults and their galls were deposited in the entomology collections of the Museo Nacional de Ciencias
Naturales, Madrid (Spain) (MNCN) and Maestría en Entomología, Universidad de Panamá (MEUP). The identification of the Quercus species was based on several key references (Burger, 1977; Breedlove, 2001), as well as comparison with materials from the collections of the University of Panama and the Smithsonian Tropical Research Institute.
SPECIMEN PREPARATION
Adult cynipids were dissected in 70% ethanol, air dried, mounted on a stub and coated with gold for observation under a scanning
electron microscope (SEM). Micrographs were taken by an EVO 40 Zeiss and FEI QUANTA 200 microscope (high vacuum technique)
for several standardized views. The forewings were mounted on slides in Euparal and were later examined under a Wild MZ8 stereo
microscope. Images of the adult habitus and gall dissections were taken with a NIKON Coolpix 4500 digital camera attached
to a Wild MZ8 stereo microscope.
TERMINOLOGY AND MEASUREMENTS
Measurements were performed with a calibrated micrometer scale attached to an ocular of the light microscope. The terminology
of morphological structures and abbreviations follows Ronquist & Nordlander (1989), Ronquist (1995), Nieves-Aldrey (2001) and Liljeblad et al. (2008). For the cuticular sculpture we follow Harris (1979). Measurements and abbreviations used include the following: the post-ocellar distance (POL) is the distance between the inner
margins of the posterior ocelli; the ocellar-ocular distance (OOL) is the distance from the outer edge of a posterior ocellus
to the inner margin of the compound eye.
ResultsTOP
DESCRIPTION OF SPECIES
Neuroterus elvisi Medianero & Nieves-Aldrey sp. n.
(Figs. 1, 2 & 7C-F)
http://urn:lsid:zoobank.org:act:0A9F90B1-5281-4FC1-BF2E-614387C8DB0A
TYPE MATERIAL. Holotype ♀ (Fig. 7C) [in Museo Nacional de Ciencias Naturales, Madrid, Spain (MNCN), card mounted. Cat. no. 2755]: PANAMA, Chiriquí, Volcán Barú,
8°47´50.8” N, 82°29´35.9” W, 1,800 – 2,070 m; ex gall on leaf of Quercus bumelioides Liebm. (Fagaceae); gall collected 08-v-2008; insect emerged v.2008, E. Medianero & J. L. Nieves leg. Paratypes: 4♂, 5♀. 3♂, 4♀, same data as holotype; 1♂, 1♀, Panamá. Boquete, Palmira, 1,093 m, ex gall Quercus lancifolia; gall collected 26.xii.2008, E. Medianero leg. In MNCN.
Additionally, 1♀, 1♂ paratypes of the type series were dissected and mounted in stubs for SEM observation (in MNCN).
|
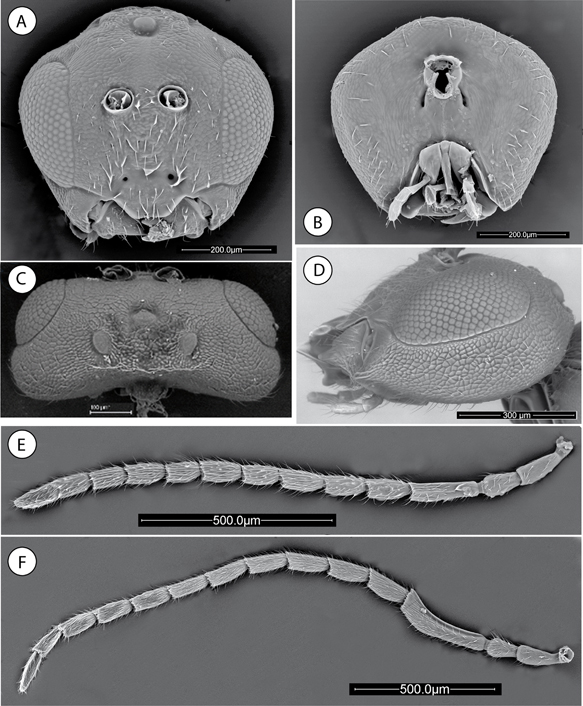 Fig. 1.—Neuroterus elvisi sp. n., adult (SEM): (A) Head, anterior view. (B) Head posterior view. (C) Head, dorsal view. (D) Head, lateral view. (E) Female antenna. (F) Male antenna. Fig. 1.—Neuroterus elvisi sp. n., adult (SEM): (A) Head, anterior view. (B) Head posterior view. (C) Head, dorsal view. (D) Head, lateral view. (E) Female antenna. (F) Male antenna.
Fig. 1.—Neuroterus elvisi sp. n. (SEM del adulto): (A) cabeza, en visión anterior. (B) Cabeza, en visión posterior. (C) Cabeza, en visión dorsal. (D) Cabeza, en visión lateral. (E) Antena de la hembra. (F) Antena del macho.
|
|
|
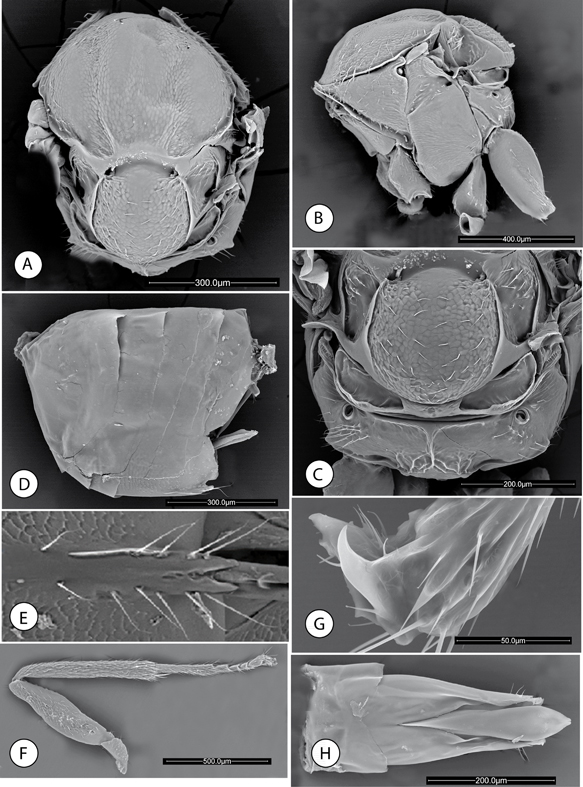 Fig. 2.—Neuroterus elvisi sp. n., adult (SEM): (A) Mesosoma, dorsal view. (B) Mesosoma, lateral view. (C) Metascutellum and propodeum. (D) Metasoma, lateral view. (E) Detail of ventral spine of hypopygium. (F) Posterior leg, coxa removed. (G) Metatarsal claw. (H) Male genitalia. Fig. 2.—Neuroterus elvisi sp. n., adult (SEM): (A) Mesosoma, dorsal view. (B) Mesosoma, lateral view. (C) Metascutellum and propodeum. (D) Metasoma, lateral view. (E) Detail of ventral spine of hypopygium. (F) Posterior leg, coxa removed. (G) Metatarsal claw. (H) Male genitalia.
Fig. 2.—Neuroterus elvisi sp. n. (SEM del adulto): (A) Mesosoma, en visión dorsal. (B) Mesosoma, en visión lateral. (C) Metascutello y propodeo. (D) Metasoma, en visión lateral. (E) Detalle de la espina ventral del hypopygio. (F) Pata posterior, coxa retirada. (G) Uña metatarsal. (H) Genitalia del macho.
|
|
DIAGNOSIS AND COMMENTS. The simple claws, distinctive malar sulcus and presence of a median propodeal carina, but lack of lateral carinae on the
propodeum, are diagnostic characters which group the new species within the Nearctic and Neotropical species of Neuroterus and differ from Palaeartic species of Neuroterus sensu lato. Neuroterus elvisi can be distinguished from the other two new species described in this paper by a combination of diagnostic characters listed
in Table 1 and as follows: predominantly yellowish coloration, notauli weak but traceable; mesoscutum with alutaceous sculpture visible,
speculum of mesopleuron smooth; antennal flagellum of female with 11 segments, median carina of propodeum conspicuous, divided
in two arms anteriorly and Rs of forewing not prolonged at apex. By the type of gall, the closet species geographically is
Neuroterus junctor Kinsey, 1938 from Mexico, although the gall of the latter is hairy, whereas the gall of N. elvisi is glabrous. Morphologically, adults of the two species are similar, but the new species differs from N. junctor in its lighter coloration, which is black in N. junctor, and mesoscutum with alutaceous sculpture (smooth in N. junctor). By the sculpture of the mesosoma and metasoma, N. elvisi resembles Neuroterus ellongatum Pujade-Villar & Melika (asexual generation), recently described from Mexico (Pujade-Villar et al., 2015), but F1 is only slightly longer that F2, and the metasoma is 2.0 times as long as high in lateral view in Neuroterus ellongatum, whereas F1 is 1.7 times as long as F2, and the metasoma is only slightly longer than high in N. elvisi. Additionally, the galls of the two species are different, developing in twigs in N. ellongatum and in leaves in N. elvisi.
Table 1.—Diagnostic morphological characters that allow the separation of the three new species described in this work.
Tabla 1.—Caracteres morfológicos diagnósticos que permiten la separación de las tres especies nuevas descritas en este trabajo.
| Morphological characters |
N. elvisi sp. n. |
N. pulchrigalla sp. n. |
N. glandiphilus sp. n. |
| Character states |
|
|
| Coloration |
Predominantly yellow |
Predominantly light brown |
Predominantly dark brown to black |
| Nº segments antennae |
13 |
13 |
13-14 |
| Pedicel: length/width |
1.6 |
1.3 |
1.5 |
| F1/F2 |
1.7 |
1.5 |
1.2 |
| Presence of placodeal sensilla on flagellum |
F1-F11 |
F3-F11 |
F2-F12 |
| Shape and sculpture of clypeus |
Trapezoidal, ventrally projected and straight margin; sculpture very weak, almost smooth. |
Square shaped, ventrally projected and straight margin; medially with weak sculpture, smooth laterally and ventrally |
Trapezoidal, ventrally projected, margin sinuate; Sculpture well marked. |
| Genae |
Slightly expanded behind eyes |
Not expanded |
Expanded |
| Mesoscutum sculpture |
Alutaceous without ridges |
Almost smooth |
Coriaceous, some longitudinal ridges |
| Notauli |
Visible through |
absent |
Indicated posteriorly |
| Mesopleuron |
Smooth in anteroposterior area |
Weakly alutaceous |
Coriaceous, well visible throughout |
| Mesoscutellum |
Posteriorly not ridged, coriaceous sculpture |
Ridged posteriorly, almost smooth |
Ridged posteriorly Very weak sculpture, rugose posterior |
| Median carina of propodeum |
Well-marked, two arms anteriorly |
Indistinct, weak |
Branched anteriorly and posteriorly |
| Forewing |
Rs enlarged apically, not quite arriving margin; rs+m incomplete |
Rs not enlarged apically, arriving margin; rs+m quite incomplete |
Rs enlarged apically, arriving wing margin; rs+m complete |
DESCRIPTION. Body length, 1.8 mm (range 1.5- 2.3 mm; N = 6) for females; 1.8 mm (range 1.5–2.2; N = 4) for males (Figs. 7C-E). Head yellowish-orange, except frons and distal part of mandibular teeth which are dark brown to blackish; mesosoma predominantly
shining brown to blackish except medial area of mesoscutum, pronotum laterally and mesopleura, which are orange to light brown.
Metasoma blackish. Scape, pedicel and first flagellomere of antennae yellowish, remaining flagellomeres light brown; legs
entirely yellowish. The males have a predominantly yellowish coloration, except the frons, lateral and anterior areas of the
mesoscutum, propodeum and metasoma (entirely), which are orange to light brown. Forewings are hyaline in both sexes.
SEXUAL FEMALE. Head (Figs. 1A-C), uniformly alutaceous, barely pubescent; in dorsal view 2.3x as broad as long. POL 1.2 times as long as OOL; posterior ocellus
separated from inner orbit of eye by 3.5 times its longest diameter (Fig. 1C). Head more or less pentagonal in anterior view, with ocellar plate raised (Fig. 1A), 1.17x as broad as high. Genae not expanded behind eyes. Vertex, frons and face uniformly alutaceous; vertex and frons with
a few sparse short setae, face with more numerous and relatively longer setae, occiput barely pubescent with sparse and shorter
setae. Clypeus trapezoidal, 2.0x as broad as high; with very weak sculpture and some long setae medially; ventral margin straight
and strongly projecting over mandibles. Anterior tentorial pits conspicuous; epistomal sulcus and clypeo-pleurostomal lines
indistinct. Malar space 0.22x as long as height of compound eye; malar sulcus distinct, ending near clypeal margin. Distance
between antennal rim and compound eye 1.3 times width of antennal socket including rim. Head, posterior view (Fig. 1B) with weak alutaceous sculpture and with some long sparse setae at external edges. Gula long; distance between occipital
and oral foramina 1.5x as long as the occipital foramen. Hypostomal sulci inconspicuous, converging at the oral foramen.
Mouthparts (Figs. 1B, 1D). Mandibles strong and exposed, right mandible with three teeth, left with two teeth. Cardo of maxilla visible, maxillary
stipes approximately 1.6 times longer than wide. Maxillary palp five-segmented. Labial palp with two visible segments.
Antennae (Fig. 1E) of moderate length, as long as 0.7 body length, with 13 segments; flagellum not broadening toward apex, with short, erect setae and elongate placodeal sensilla visible on all flagellar segments. Relative length/width of antennal segments: 20/9:16/10:30/9:18/10:17/10:20/10:16/9:17/10:15/10:13/10:15/10:15/10:10/9. Pedicel, 1.6x as long as broad, 0.8 times as long as scape; F1 1.7x as long as F2 (Fig. 1E); F11 2x as long as broad and 1.3 times as long as F10 (Fig. 1E).
Mesosoma short; 1.2x as long as broad in dorsal view (Fig. 2A), slightly longer than high in lateral view and with dorsal margin strongly convex (Fig. 2B). Pronotum very short medially in frontal view, with weak alutaceous sculpture in lateral view and barely pubescent.
Mesonotum. Mesoscutum (Fig. 2A), delicately coriaceus, shiny, glabrous except for a group of sparse short setae anteromedially. Notauli shallowly marked
but visible, more clearly in posterior one third of mesoscutum; anteroadmedian signa and parapsidial signa absent, indicated
only by delicate sculpture (Fig. 2A). Median mesoscutal impression absent. Transscutal fissure absent. Scutellum rounded (Fig. 2A), approximately 0.5 times as long as mesoscutum, delicately coriaceous, barely pubescent, dorsal surface with a distinct
sharp margin posterolaterally, posterior margin not emarginate. Scutellar foveae in form of transverse deep groove, smooth
and shining. Scutellum, barely overlapping the dorsellum posteriorly in lateral view (Fig. 2B). Mesopleuron (Fig. 2B) weakly alutaceous, except for the smooth postero dorsal area (speculum); barely pubescent with sparse shorter setae in mesopleural
triangle.
Metanotum (Fig. 2C). Metapectal-propodeal complex. Metapleural sulcus reaching posterior margin of mesopectus at about two thirds distance from
ventral margin (Fig. 2B). Metascutellum with weak rugose sculpture; metanotal trough smooth and glabrous. Propodeum almost smooth; lateral propodeal
carinae absent; medial carina present, divided in two branches anteriorly and posteriorly in some longitudinal carinae (Fig. 2C). Median propodeal area smooth and pubescent on the sides
Legs. Metafemur 3.6x as long as broad; metatibia 1.3x as long as combined length of metatarsomeres (Fig. 2F). Metatarsal claws simple, without a basal lobe or tooth (Fig. 2G).
Forewing (Fig. 7F). 1.3 times as long as body, hyaline, setose, veins dark brown to black. Radial cell 4x as long as broad, open along anterior
margin, areolet triangular, closed and distinct; R1 oriented obliquely to anterior margin of wing. Rs slightly expanded at
apex. R1 reaching margin of wing, Rs ending close to wing margin. M nearly straight, not reaching wing margin. Rs+M incomplete
reaching basalis at its mid-height. First abscissa of radius (2r) slightly angulate in the middle. Basal cell setose; costal
cell conspicuously setose. Apical margin of wing with a fringe of long setae.
Metasoma (Fig. 2D). Short, as long as mesosoma, 1.2x as long as high, in lateral view. Tergites smooth and shining dorso-laterally, T4-T7 alutaceous
ventrally (Fig. 2E). T3 with a group of sparse short setae anteromedially. Projecting part of hypopygial spine, beyond attachment of lateral
flap, relatively short (Fig. 2E); approximately 1.1 times as long as basal height of the spine; lateral margins of hypopygial spine with long setae projecting
over apical end of the spine.
MALE. Besides the lighter body, antennae and leg coloration (Fig. 7D), differs from the female as follows: antennae (Fig. 1F) with 14 segments; F1 2.3x as long as F2; F1 modified, curved and flattened and slightly expanded distally. Mesoscutum without
alutaceous sculpture, smooth and shining. Genitalia. Phallus (Fig. 2H). Apical part of aedeagus moderately expanded subapically; length of paramere short, not reaching beyond digitus; basidorsal
margin of parameral plates only weakly incised medially. Apical margin of basal ring incised.
GALL (Figs. 8F-H). Small, irregular swellings of the leaves, with smooth and bare surface. Polythalamous, developing in petiole and midrib
and engulfing leaf lamina. The galls are light green when fresh but dark green when mature and brown when old. Diameter 10
to 18 mm. Galls are relatively common on Quercus bumelioides at the Volcán Barú site. The gall most closely resembles that of Neuroterus junctor from Mexico.
DISTRIBUTION. N. elvisi was found at 1,800 m above sea level at Volcán Barú, Chiriquí Province, Panama.
ETYMOLOGY. Named after Elvis Segundo for his help in field work and oak gall wasps samplings in the mountains of Panama.
BIOLOGY. Sexual generation. Galls formed in the leaves of Q. bumelioides mature in May and insects emerge soon thereafter in the same month. Although the asexual generation is unknown, we have circumstantial
evidence that they are similar to those of the sexual generation but develop in early November (Fig. 8H) during the rainy season when new Q. bumelioides leaves begin to appear. The similarity between galls and insects of the two alternating generations in the Nearctic species
of Neuroterus has been mentioned by Kinsey (1923).
Neuroterus pulchrigalla Medianero & Nieves-Aldrey sp. n. (Figs. 3, 4 & 7A-B)
http://urn:lsid:zoobank.org:act:B41783B3-6490-42B8-8471-2F6E71B2A861
TYPE MATERIAL. Holotype ♀ (Fig. 7A) [in Museo Nacional de Ciencias Naturales, Madrid, Spain (MNCN), card mounted. Cat. no. 2756]: PANAMA, Boquete, El Salto,
8°47´32.8” N, 82°27´32.9” W, 1,431 m; ex gall on leaf of Quercus bumelioides Liebm. (Fagaceae); gall collected 7.v-2008; insect emerged v.2008, Medianero & Nieves-Aldrey leg. Paratypes: 4♀, 1♀ same data as holotype; 1♀ same data as holotype, but collected 30.xii.2008 E. Medianero leg. 2♀ Panama, same data
as holotype but gall collected 30.i.2008. Additionally, 2♀ paratypes of the type series were dissected for SEM observation
(in MNCN).
|
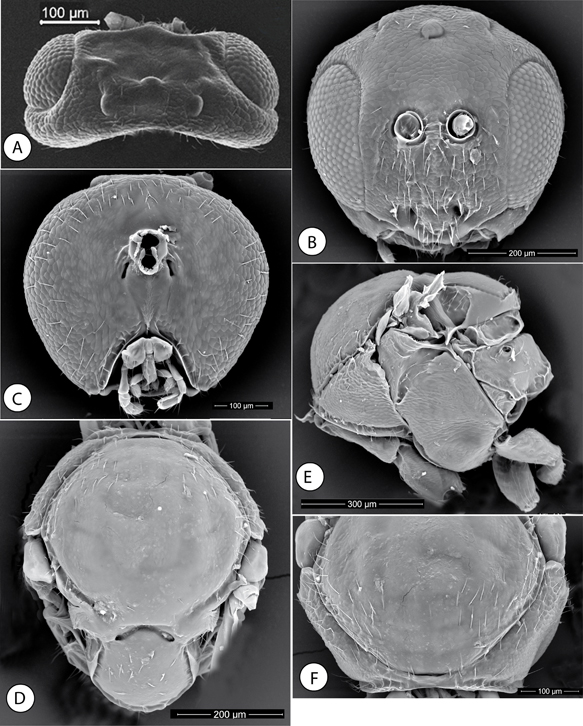 Fig. 3.—Neuroterus pulchrigalla sp. n., adult (SEM): (A) Head, dorsal view. (B) Head, anterior view. (C) Head, posterior view. (D) Mesosoma, dorsal view. (E) Mesosoma, lateral view. (F) Pronotum, anterior view. Fig. 3.—Neuroterus pulchrigalla sp. n., adult (SEM): (A) Head, dorsal view. (B) Head, anterior view. (C) Head, posterior view. (D) Mesosoma, dorsal view. (E) Mesosoma, lateral view. (F) Pronotum, anterior view.
Fig. 3.—Neuroterus pulchrigalla sp. n. (SEM del adulto): (A) cabeza, en visión dorsal. (B) Cabeza, en visión anterior. (C) Cabeza, en visión posterior. (D) Mesosoma, en visión dorsal. (E) Mesosoma, en visión lateral. (F) Pronoto, en visión anterior.
|
|
|
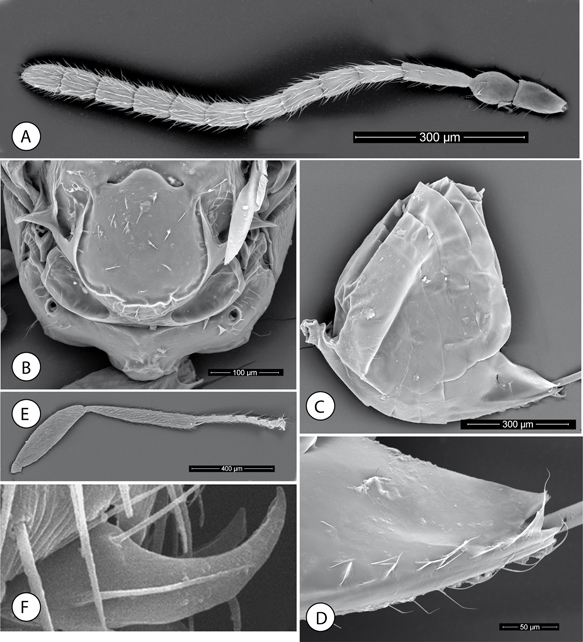 Fig. 4.—Neuroterus pulchrigalla sp. n. Adult (SEM): (A) Antenna. (B) Propodeum. (C) Metasoma, lateral view. (D) Detail of ventral spine of hypopygium. (E) Posterior leg, coxa removed. (F) Metatarsal claw. Fig. 4.—Neuroterus pulchrigalla sp. n. Adult (SEM): (A) Antenna. (B) Propodeum. (C) Metasoma, lateral view. (D) Detail of ventral spine of hypopygium. (E) Posterior leg, coxa removed. (F) Metatarsal claw.
Fig. 4.—Neuroterus pulchrigalla sp. n. (SEM del adulto): (A) Antena. (B) Propodeo. (C) Metasoma, en vision lateral. (D) espina ventral del hipopigio, en visión ventral. (E) Pata posterior, sin la coxa. (F) Uña metatarsal.
|
|
DIAGNOSIS AND COMMENTS. Neuroterus pulchrigalla is similar to Neuroterus elvisi in many morphological diagnostic characters. Besides the light brown coloration in N. pulchrigalla, the two species can be readily separated as follows (see Table 1): Antennal pedicel only 1.3x as long as broad (1.6x in N. elvisi); placodeal sensilla present only on flagellar segments 3 to 11, while present in all the flagellomeres in N. elvisi; genae not expanded behind eyes; clypeus evidently not trapezoidal; mesoscutum almost smooth and notauli invisible; mesopleuron
entirely alutaceous, mesoscutellum smooth, median propodeal carina not marked and Rs of forewing not apically enlarged. The
gall of the new species has a distinctive appearance (Figs. 8A-E). Among the Nearctic species of Neuroterus, including the species described from Mexico, only the gall of Neuroterus argentatus Weld, 1944, described from Arizona on Quercus gambelii, has some resemblance, but the galls of N. argentatus are different in shape and covered by radiating silvery hairs. Furthermore, the morphology of the adults is also different,
mainly with respect to body coloration and the relative length of flagellomeres F1/F2.
GENERAL DESCRIPTION. Body length, 1.41 mm (range 1.2-1.75 mm; N=12) for females. Female body predominantly dark brown. Scape, pedicel and F1 yellow, remaining flagellomeres dark yellowish.
Legs predominantly brown, with coxae distally, trocanters, distal femorae, tibiae (except metatibia) and all tarsi yellowish.
Forewings hyaline with veins brown.
ASEXUAL FEMALE. Head, uniformly alutaceous, barely pubescent; in dorsal view approximately 2.4x as broad as long (Fig. 3A). POL 1.67x longer than OOL; posterior ocellus separated from inner orbit of eye by 2.3 times its longest diameter (Fig. 3A). Genae not expanded behind eyes. Head oval elongate in anterior view (Fig. 3B), about as high as broad. Vertex, frons and face uniformly alutaceous; vertex and frons without setae, face with relatively
long white setae. Clypeus trapezoidal, 1.6x wider than high, shining, moderately pubescent, ventral margin straight and projecting
over mandibles; median area of clypeus with weak alutaceous sculpture; the lateral and the ventral projected areas smooth.
Anterior tentorial pits conspicuous; epistomal sulcus and clypeo-pleurostomal lines indistinct. Malar space 0.2x the height
of compound eye, with a distinctive, complete and well-impressed malar sulcus. Distance between antennal rim and compound
eye 1.3x the width of antennal socket including rim. Ocellar plate slightly raised. Head, posterior view (Fig. 3C) with weak alutaceous sculpture and some setae on outer areas. Gula long; distance between occipital and oral foramina 1.5x
as high as the occipital foramen. Hypostomal sulci visible, converging at the oral fosa. Without an occipital carina.
Mouthparts. Mandibles strong and exposed, right mandible with three teeth, left with two teeth. Cardo of maxilla not visible,
maxillary stipes 1.4x as long as broad. Maxillary palp five-segmented. Labial palp with two visible segments.
Antennae (Fig. 4A) of moderate length, as long as 1/2 body length, with 13 segments; flagellum slightly broadening toward apex, with short, erect setae and elongate placodeal sensilla visible only on flagellar segments 3-11. Relative length/width of antennal segments as: 21/12:20/15:30/8:20/8:16/10:19/10:17/10:17/10:16/11:17/11:17/11:15/11:17/12. Pedicel, globose, 1.3x as long as broad, 0.6x as long as F1; F1 1.5x as long as F2 (Fig. 4A); F11 1.4x as long as broad and slightly longer than F10.
Mesosoma short, 1.2x as long as broad in dorsal view (Fig. 3D), 1.1x as long as high in lateral view and with dorsal margin strongly convex (Fig. 3E). Pronotum in frontal view very short medially, 0.08x as long as lateral distance of pronotum (Fig. 3F), pronotal plate indistinct, with alutaceous sculpture in lateral view, and barely pubescent (Fig. 3E).
Mesonotum. Mesoscutum (Fig. 3D), virtually smooth, although with obsolete alutaceous sculpture visible in some areas, glabrous except for a group of sparse
short setae anterolaterally. Notauli and median mesoscutal impression absent; anteroadmedian signa and parapsidial signa absent.
Transscutal fissure absent. Scutellum rounded, approximately 0.5x as long as mesoscutum, almost smooth and barely pubescent,
dorsal surface with a distinct sharp margin posterolaterally, posterior margin not emarginate. Scutellar foveae in form of
transverse deep, smooth and shining, inverted V-shaped groove. Scutellum not overlapping the metascutelum posteriorly in lateral
view (Fig. 3E). Mesopleuron (Fig. 3E) weakly alutaceous and glabrous on its entire surface; ventral margin of mesopleural triangle somewhat interrupted in the
middle.
Metanotum (Fig. 4B). Metapectal-propodeal complex. Metapleural sulcus reaching posterior margin of mesopectus at about two thirds distance from
ventral margin (Fig. 3E). Metascutellum with weak rugose sculpture; metanotal trough smooth and glabrous. Propodeum almost smooth; lateral propodeal
carinae absent; a medial, weakly marked carina is visible (Fig. 4B). Median propodeal area smooth and pubescent on the sides.
Legs. Metafemur 3x as long as broad; metatibia 1.3x as long as combined length of metatarsomeres (Fig. 4E). Metatarsal claws simple, without a basal lobe or tooth (Fig. 4F).
Forewing (Fig. 7B). 1.3x as long as body, hyaline, setose, veins dark brown to black. Radial cell 4x as long as broad, open along anterior
margin, areolet triangular, closed and distinct; R1 oriented obliquely to anterior margin of wing. Rs not expanded at apex;
R1 and Rs reaching margin of wing, Rs+M incomplete reaching basalis at its mid-height. First abscissa of radius (2r) slightly
angulate in the middle. Basal cell and costal cell setose. Apical margin of wing with a fringe of long setae.
Metasoma (Fig. 4C). As long as head + mesosoma combined; as long as high in lateral view. Tergites, smooth and shining dorso-laterally (Fig. 4C). T3 with a group of sparse short setae anteromedially. Projecting part of hypopygial spine short, about as long as broad
(Fig. 4D); lateral margins of hypopygial spine with long setae, the subapical ones projecting over apical end of the spine.
GALL (Figs. 8A-E). Galls have a cylindrical shape, measuring 5 x 2 mm. the gall surface is covered with long dense yellowish hairs. Internally,
two parts are visible; an ovoid larval cell at the attachment of the gall on the leaf and an apical empty part (Fig. 8D). The galls grow isolated or more frequently grow in close clusters formed by 2-10 galls on the midrib of Quercus bumelioides leaves (Figs. 8A-C). The galls are yellow when fresh, orange when mature and brown when old. Galls are relatively abundant on Quercus bumelioides at the Volcán Barú site in Panama.
DISTRIBUTION. Neuroterus pulchrigalla was found between 1,431-1,800 m above sea level at Volcán Barú and El Salto, Chiriquí Province, Panama. We have found galls
of this species in the same type locality type as the other new species described in this paper from Panama.
ETYMOLOGY. Named after the pretty, brilliantly colored leaf galls induced by this species.
BIOLOGY. Only the asexual generation of Neuroterus pulchrigalla is known, inducing galls on Q. bumelioides leaves. The galls are found between January and March, during the dry season, when new Q. bumelioides leaves begin to mature. The adult insects emerge from mature galls in February or May.
Neuroterus glandiphilus Nieves-Aldrey & Medianero sp. n.
(Figs. 5, 6, 7G-H & 9)
http://urn:lsid:zoobank.org:act:430EEB3E-FF4E-4407-8950-E605BF278F69
TYPE MATERIAL. Holotype. 1♀ (Fig. 7G) (in Museo Nacional de Ciencias Naturales, Madrid, Spain (MNCN), card mounted. Cat. nº 2757). COSTA RICA, Cartago province,
Truchas Selva Madre, 09º 40’ 27.09 2” N, 83º 52’ 50.53” W, 2,535 m; ex galls on acorns of Quercus bumelioides Liebm. (Fagaceae), galls collected 05.i.2014, insect emerged 08.i.14, J. L. Nieves leg. Paratypes. 1♂, 7♀ same data as holotype. Paratypes in MNCN. 2♀ paratype of the type series were dissected for SEM observation.
|
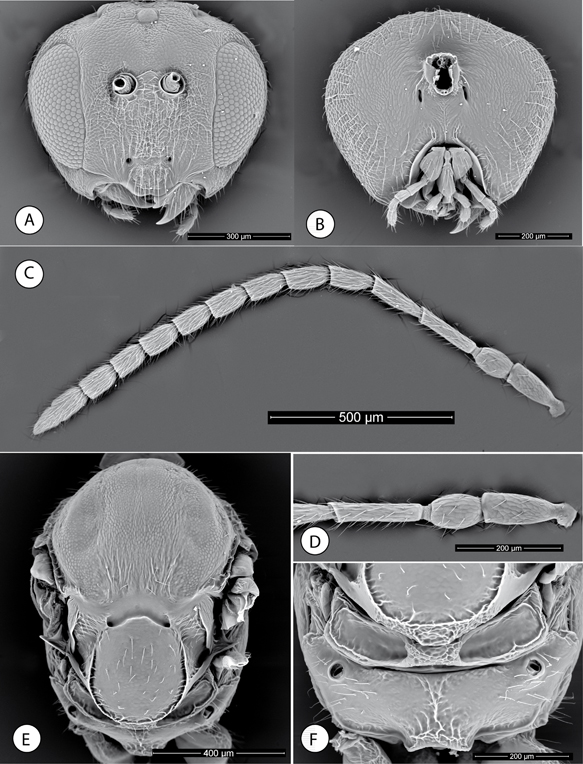 Fig. 5.—Neuroterus glandiphilus sp. n. Adult (SEM): (A) Head, anterior view. (B) Head, posterior view. (C) Antenna. (D) Detail of basal antennomeres. (E) Mesosoma, dorsal view. (F) Propodeum. Fig. 5.—Neuroterus glandiphilus sp. n. Adult (SEM): (A) Head, anterior view. (B) Head, posterior view. (C) Antenna. (D) Detail of basal antennomeres. (E) Mesosoma, dorsal view. (F) Propodeum.
Fig. 5.—Neuroterus glandiphilus sp. n. (SEM del adulto): (A) Cabeza, en visión anterior. (B) cabeza, en visión posterior. (C) Antena. (D) Detalle de los antenómeros basales. (E) Mesosoma, en visión dorsal. (F) Propodeo.
|
|
|
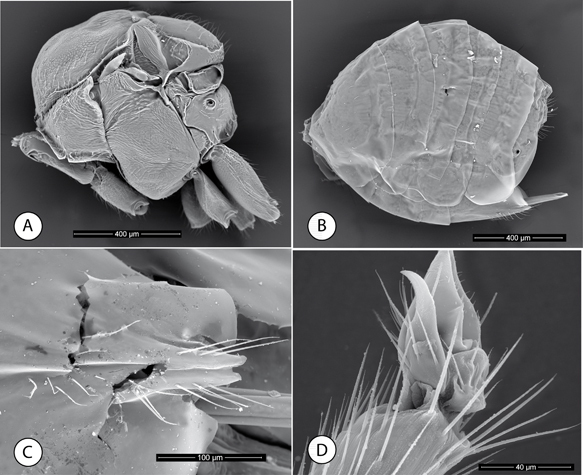 Fig. 6.—Neuroterus glandiphilus sp. n. Adult (SEM): (A) Mesosoma, lateral view. (B) Metasoma, lateral view. (C) Ventral spine of hypopygium, ventral view. (D) Metatarsal claw. Fig. 6.—Neuroterus glandiphilus sp. n. Adult (SEM): (A) Mesosoma, lateral view. (B) Metasoma, lateral view. (C) Ventral spine of hypopygium, ventral view. (D) Metatarsal claw.
Fig. 6.—Neuroterus glandiphilus sp. n. (SEM del adulto): (A) Mesosoma, en vision lateral. (B) Metasoma, en vision lateral. (C) Espina ventral del hipopigio. (D) Uña metatarsal.
|
|
DIAGNOSIS AND REMARKS. Neuroterus glandiphilus differs from N. elvisi and N. pulchrigalla in the diagnostic characters and character states shown in Table 1. N. glandiphilus is readily distinguished by the 13/14 segmented antenna; with F1 only 1.2x as long as F2; mesoscutum with visible coriaceous
sculpture, including some longitudinal ridges on the postero medial area; the dark brown to black coloration, mesopleuron
entirely coriaceous, and the median propodeal carina branched anteriorly and posteriorly are additional distinguishing features.
We know of only one species of Neuroterus in the American continent galling acorns, Neuroterus cupulae Kinsey, 1922, later considered an asexual generation synonym of Neuroterus quercicola var. pacificus Kinsey (Kinsey, 1923; Weld, 1952), a species distributed in California. However, the gall of N. cupulae differs from the gall of N. glandiphilus in its smaller size and position in the acorn cup.
DESCRIPTION. Body length 2.2 mm (range 2-2.5; N = 5) for females; 2.2 mm (N = 1) for males. Head, mesosoma and metasoma of female black,
Mandibles light brown. Antenna with scape, pedicel and first flage-llomere light brown; remaining flagellomeres dark brown.
Forewing hyaline, with brown veins. Legs brown except coxae, proximal femur, distal tibiae and last tarsomere dark brown to
blackish. Males have a similar coloration.
SEXUAL FEMALE (Fig. 7G). Head 2.4x as broad as long in dorsal view, slightly broader than mesosoma in dorsal view. Temples expanded behind compound
eye. POL 1.6x OOL; posterior ocellus separated from inner orbit of eye by 2.6x its longest diameter. Head 1.15x as broad as
high in anterior view (Fig. 5A); genae slightly expanded behind eye. Face with sparse long setae, absent in frontal area. Face and frons with weak coriaceous
sculpture. Clypeus distinct, with tighter coriaceous sculpture, its ventral margin sinuate and projecting over mandibles (Fig. 5A). Anterior tentorial pits conspicuous; epistomal and clypeo-pleurostomal lines not marked. Malar space 0.26x height of compound
eye. Malar sulcus conspicuously well marked. Toruli situated slightly above mid-height of compound eye; distance between antennal
rim and compound eye 1.2x width of antennal socket including rim. Head in posterior view with weak coriaceous sculpture and
sparse setae around external edges (Fig. 5B). Gula short; distance between occipital foramen and oral foramen as long as the height of the occipital foramen. Posterior
tentorial pits clearly visible, buttonhole-like; hypostomal sulci visible, converging and slightly separated at the oral foramen.
Mouthparts (Fig. 5B). Mandibles partially exposed in anterior view of head; right mandible with three teeth; left with two teeth. Cardo of maxilla
hardly visible, maxillary stipes about as long as broad. Maxillary palp five-segmented. Labial palp with two visible segments.
Antenna 0.6x as long as body; with 13-14 segments (13 and 14 sometimes incompletely divided) (Fig. 5C); flagellum slightly broadening towards apex; with erect setae and placodeal sensilla visible on flagellar segments F2–F12, arranged in one row of 1-3 sensillae on each flagellomere. Relative length/width of antennal segments: 20/9:15/10:26/6:22/7:17/8:
17/8:16/8:15/9:15/9:16/9:14/9:15/9:14/9:11/7. Pedicel 1.5x as long as broad; F1 1.2x as long as F2. Last flagellomere 1.5x
as long as broad, 0.8x as long as F11.
Mesosoma short; in dorsal view (Fig. 5E) 1.4x as long as broad, slightly longer than high in lateral view and with dorsal margin convex. Pronotum medially very short,
without pronotal plate, laterally with alutaceous sculpture, with some setae dorsally and some longitudinal rugae at posterior
margin (Fig. 6A). Mesoscutum (Fig. 5E) with weak coriaceous-alutaceous sculpture; medially, towards transcutal fissure, some longitudinal rugae visible jointly
with a few long setae. Notauli traceable but indistinct, not marked, without clear limits. Median mesoscutal impression almost
invisible. Anteroadmedian signa scarcely visible; parascutal impressions not visible. Transscutal fissure absent, marked as
a deep depression without clear limits between posterior margin of mesoscutum and posterior margins of scutellar foveae. Scutellar
foveae united into a smooth and shining broad depression only limited by a posterior margin. (Fig. 5E). Mesoscutellum oval, flat, with outer raised margins, almost smooth and scarcely pubescent. Mesopleuron (Fig. 6A) with visible coriaceous sculpture and almost glabrous. An interrupted line marking ventral margin of mesopleural triangle
visible.
Metapectal-propodeal complex. Metapleural sulcus meeting posterior margin of mesopectus at middle point, relative to the posterior
subalar pit. Metascutellum with rugose sculpture; metanotal trough smooth and glabrous. Propodeum almost smooth; lateral
propodeal carinae absent; medial carine present, which is branched anteriorly and subdivided posteriorly in several short
longitudinal carinae (Fig. 5F). Median propodeal area smooth and pubescent on the sides. Nucha dorsally with longitudinal ridges.
Legs. Metatarsal claw (Fig. 6D) simple, without secondary basal lobe or tooth.
Forewing (Fig. 7H). 1.3x as long as body; conspicuously setose. Radial cell 4.3x as long as broad, open along anterior margin; R1 and Rs reaching anterior margin of wing; R1 oriented obliquely to anterior margin of wing. Rs slightly expanded at apex; Rs+M distinct, reaching
basalis slightly below middle part of its height. Medial and cubital veins visible; areolet large, triangular. Basal cell
with some setae; costal cell heavily setose. Apical margin of wing with a fringe of long setae.
Metasoma (Fig. 6B). Slightly shorter than head + mesosoma. T2 0.4x as long as metasoma. Metasomal tergites glabrous, smooth and shining. Projecting part of hypopygial spine short (Fig. 6C); approximately 2x as long as high; hypopygial spine with some long erect setae in ventral view, the apical ones overlapping
apex of spine.
MALE. Similar to female in coloration and the majority of morphological characters. Differs from female as follows: Antennae with
14 clearly separate segments; F12 as long as F11. Mesoscutum without alutaceous sculpture, smooth and shining.
DISTRIBUTION. The collecting site of the type material of this new species is located in the tropical montane cloud forests of the Cordillera of Talamanca in Costa Rica at an altitude above 2,500 m (Fig. 9B). The host tree, Quercus bumelioides Liebm. (=Q. copeyensis CH Mull.), is dominant in the cloud forest of this region of Costa Rica (Kappelle, 1996). The host tree is also distributed in other areas of Central America and southern Mexico, a large area that represents the potential distribution of their associated gall wasps. The new species is the first oak gall wasp (Cynipini) of the genus
Neuroterus described from Costa Rica.
GALL (Figs. 9C-F). The galls develop inside acorns. They form a cluster of aggregated and joined chambers within the cotyledons and wall of
the acorn. Individual galls consist of an oval larval cell surrounded by tissue that is pointed at the apex. Individual galls
are grouped radially, appearing as orange segments. Affected acorns do not develop normally and remain stunted inside the
acorn cup. Mature galls have woody, hard walls. The galled acorns measure 7 to 14 mm.
ETYMOLOGY. Named after the acorn gall induced by this species.
BIOLOGY. Sexual generation. The adults emerged from the galls in the laboratory a few days after being collected, in early January.
The asexual generation is unknown. One damaged female, presumably asexual according its morphology, was collected from different
small galls in the acorn cup, which may be the alternating form of N. glandiphilus, but this hypothesis must be confirmed with additional collected galls and reared adult insects.
|
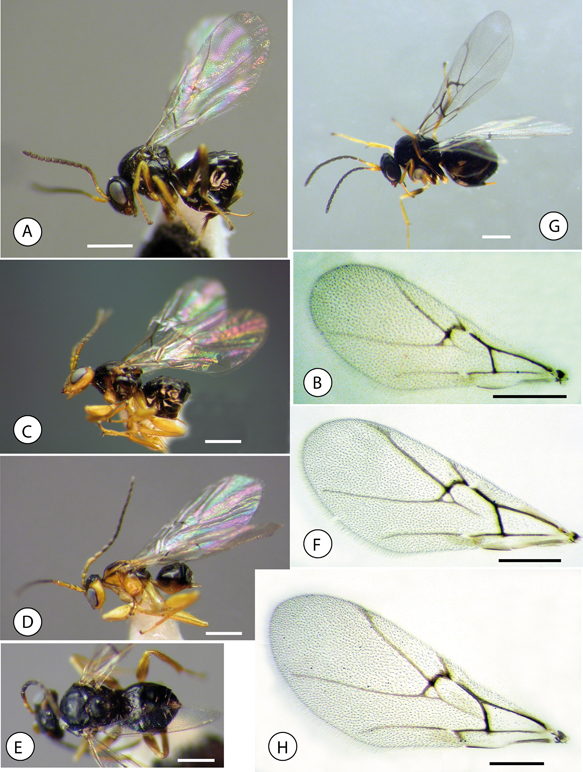 Fig. 7.—Habitus and forewings; (A) Neuroterus pulchrigalla sp. n., habitus, female. (B) Forewing. (C) Neuroterus elvisi sp. n., habitus female. (D) habitus, male. (E) habitus female, dorsal view. (F) forewing. (G) Neuroterus glandiphilus sp. n., habitus female. (H) forewing. Scale bar = 0.5 mm. Fig. 7.—Habitus and forewings; (A) Neuroterus pulchrigalla sp. n., habitus, female. (B) Forewing. (C) Neuroterus elvisi sp. n., habitus female. (D) habitus, male. (E) habitus female, dorsal view. (F) forewing. (G) Neuroterus glandiphilus sp. n., habitus female. (H) forewing. Scale bar = 0.5 mm.
Fig. 7.—Habitus y alas anteriores: (A) Neuroterus pulchrigalla sp. n., habitus de la hembra. (B) Ala anterior. (C) Neuroterus elvisi sp. n., habitus de la hembra. (D) habitus del macho. (E) habitus de la hembra, en visión dorsal. (F) ala anterior. (G) Neuroterus glandiphilus sp. n., habitus de la hembra. (H) ala anterior. Barra de la escala = 0.5 mm.
|
|
|
 Fig. 8.—Galls: (A-C) Neuroterus pulchrigalla sp. n. on leaves of Quercus bumelioides from Volcán Barú (Panamá). (D) Section of a gall. (E) Solitary gall on leaf of Q. bumelioides from Costa Rica. (F-G) Neuroterus elvisi sp. n. on leaves of Q. bumelioides. (H) Section of a gall. Scale bar = 0.5 mm. Fig. 8.—Galls: (A-C) Neuroterus pulchrigalla sp. n. on leaves of Quercus bumelioides from Volcán Barú (Panamá). (D) Section of a gall. (E) Solitary gall on leaf of Q. bumelioides from Costa Rica. (F-G) Neuroterus elvisi sp. n. on leaves of Q. bumelioides. (H) Section of a gall. Scale bar = 0.5 mm.
Fig. 8.—Agallas: (A-C) Neuroterus pulchrigalla sp. n. en hojas de Quercus bumelioides en Volcán Barú (Panamá). (D) Sección de una agalla. (E) Agalla solitaria en la hoja de Q. bumelioides colectada en Costa Rica. (F-G) Neuroterus elvisi sp. n. en hojas de Q. bumelioides. (H) Sección de una agalla. Barra de la escala = 0.5 mm.
|
|
|
 Fig. 9.—Galls of Neuroterus glandiphilus sp. n.: (A) Old gall on acorn of Q. bumelioides. (B) Aspect of the host plant. (C) Acorn showing a gall. (D-E) Section of an acorn with galls. (F) An adult female emerging from a gall. Scale bar = 1 mm. Fig. 9.—Galls of Neuroterus glandiphilus sp. n.: (A) Old gall on acorn of Q. bumelioides. (B) Aspect of the host plant. (C) Acorn showing a gall. (D-E) Section of an acorn with galls. (F) An adult female emerging from a gall. Scale bar = 1 mm.
Fig. 9.—Agallas de Neuroterus glandiphilus sp. n.: (A) Agalla vieja en la bellota de Q. bumelioides. (B) Aspecto de la planta hospedadora. (C) Bellota infectada con una agalla. (D-E) Sección de una bellota con agallas. (F) hembra adulta que acaba de emerger de una agalla. Barra de la escala = 1 mm.
|
|
|
 Fig. 10.—Galls of unidentified species of Neuroterus from Panama: (A) Gall on midrib of a leaf of Quercus lancifolia from Renacimiento. (B) Twig gall showing larvae on Quercus bumelioides from Volcán Barú. (C) Catkin gall on Quercus lancifolia from Volcán Barú. (D) Solitary gall under leaf of Quercus bumelioides from Volcán Barú. (E) Galls on leaf of Q. bumelioides from Volcán Barú (F) Leaf gall on Quercus bumelioides from Volcán Barú. Fig. 10.—Galls of unidentified species of Neuroterus from Panama: (A) Gall on midrib of a leaf of Quercus lancifolia from Renacimiento. (B) Twig gall showing larvae on Quercus bumelioides from Volcán Barú. (C) Catkin gall on Quercus lancifolia from Volcán Barú. (D) Solitary gall under leaf of Quercus bumelioides from Volcán Barú. (E) Galls on leaf of Q. bumelioides from Volcán Barú (F) Leaf gall on Quercus bumelioides from Volcán Barú.
Fig. 10.—Agallas de especies indeterminadas de Neuroterus de Panamá: (A) Agalla en el nervio medio de una hoja de Quercus lancifolia colectada en Renacimiento. (B) Agalla en las ramitas de Quercus bumelioides de Volcán Barú, (C) Agalla en amentos de Quercus lancifolia en Volcán. (D) Agalla solitaria en el envés de una hoja de Quercus bumelioides en Volcán Barú. (E) Agallas en la hoja de Q. bumelioides en Volcán Barú (F) Agalla foliar sobre Quercus bumelioides de Volcán Barú.
|
|
DiscussionTOP
Melika et al. (2010) proposed the current accepted generic circumscription and limits of Neuroterus. However, the phylogenetic relationships and generic limits between Neuroterus and closely related genera, especially the new related genera, the Oriental Cycloneuroterus and the Nearctic species of Neuroterus remain uncertain and thus require more thorough phylogenetic studies.
The Nearctic species of Neuroterus have not been revised since Kinsey (1923). Tang et al. (2011) presented a combination of morphological characters that do not fit the concept of the genus Neuroterus defined by Melika et al. (2010), concluding that the taxonomic status of many Nearctic species is unclear and needing thorough revision. Pujade-Villar et al. (2015) argued that Nearctic Neuroterus is a polyphyletic group as currently defined and suggested that new genera will be established. However, there are no phylogenetic
studies to support this hypothesis. Beyond speculation, there is an urgent need for a complete revision of Neuroterus and related genera, including the Nearctic and Neotropical species.
The new species described here present some important morphological diagnostic characters that do not fit with those defining
Neuroterus given by Melika et al. (2010). The anomalous characters are mainly the simple tarsal claws, antenna with 11/12 flagellomeres and the sculpture of propodeum.
However, we describe the species within the old concept of Neuroterus sensu Kinsey 1923, until the uncertainties about the placement of the American species of Neuroterus are resolved in a comprehensive revision of all the included species.
In this paper, the genus Neuroterus is recorded in Central America and so from the Neotropical Region for the first time. Along with the three new species described
here, we found evidence of at least six distinctive galls with cynipid gall inducers, which likely represent additional new
undescribed species (Fig. 10). We do not describe these species here, however, because of the incompleteness of the studied material. Regardless, the
available evidence clearly shows that a rich fauna of Neuroterus species exists in the tropical montane oak forests of Central America, estimated at approximately 10-20 species (Medianero
& Nieves-Aldrey, unp.). With more than 10 Quercus species as potential hosts, further studies and field work are necessary to reveal their undescribed associated cynipid fauna,
not only of Neuroterus but also of other oak gall wasp (Cynipini) genera. South of Panama, there is still a possibility of finding the genus in
Colombia, but no species of Neuroterus have been found in Colombia despite some field work in recent years. In comparison, only nine Neuroterus species have been described from Mexico (Kinsey, 1938; Pujade-Villar et al., 2015, 2016), a comparatively low number, surely reflecting poor sampling considering the 161 species of Quercus recorded from México, including 109 endemic species (Valencia-A, 2004), with presence of many species of white oaks that are their potential hosts.
HOST PLANT ASSOCIATIONS
The three new species described here induce galls on Quercus bumelioides and less frequently on Q. lancifolia, two species of white oaks belonging to the Quercus section of Quercus (Govaerts & Frodin, 1998). These results do not contradict the host association of most Nearctic species of Neuroterus, which are strictly confined to “white oaks” according to Kinsey (1923), with the exception of a more recently described species, N. chrysolepis Lyon, 1984, which is associated with the Protobalanus group of Quercus (Melika & Abrahamson, 1997). We did not find galls of Neuroterus species on Quercus salicifolia (section Lobatae, red oaks) sampled in Panama.
With regard to life cycles of the new species, two correspond to sexual generations, while the third is described from the
asexual generation. However, we found circumstantial evidence in N. elvisi and N. glandiphilus indicating that they likely have alternating generations on the same host Quercus species and thus have heterogonic life cycles. Kinsey (1923) observed that the Nearctic species of Neuroterus included in the subgenus Dolichostrophus had largely identical alternating generations. According Tang et al. (2011), of the 59 species of Nearctic Neuroterus, 30 species are known only from their sexual generation, and the connection between the asexual and sexual generation has
been established experimentally in only three species.
AcknowledgmentsTOP
We are indebted to Elvis Segundo for assistance with field sampling and to Jorge Ceballos (STRI), Laura Tormo, and Alberto
Jorge (MNCN) for technical assistance in the production of the SEM photographs. We wish to thank George Melika and one anonymous
reviewer for their valuable comments to improve the manuscript. E. M. was funded by the Sistema Nacional de Investigación
of the SENACYT in Panama, the University of Panama and research project 52-2016-4-ITE1505. JLNA was supported in part by research
projects CGL2010-15786/BOS and MINECO/FEDER, UE) CGL2015-66571-P.
ReferencesTOP
| ○ |
Breedlove, D., 2001. Fagaceae. In: W. D. Stevens, U. C., Ulloa, A. Pool & O. M. Montiel [eds.]. Flora de Nicaragua. Monographs in Systematic Botany. Missouri Botanical Garden, 85(2): 1076–1084.
|
| ○ |
Burger, W., 1977. Fagaceae. In: W. Burger (ed.). Flora Costaricensis. Fieldiana: Botany, 40: 59–82
|
| ○ |
Burks, B. D., 1979. Superfamily Cynipoidea. In: K. V. Krombein, P. D. Hurd Jr., D. R. Smith & B. D. Burks (eds.). Catalog of Hymenoptera in America of North of Mexico. Volume 1. Symphyta and Apocrita. Smithsonian Institution Press. Washington: 1045–1107.
|
| ○ |
Csóka, G., Stone, G. & Melika, G., 2005. Biology, Ecology, and Evolution of gall-inducing Cynipidae. In: A. Raman, C. Schaefer & T. Withers (eds.). Biology, ecology, and evolution of gall-inducing Arthropods. Science Publishers. Enfield: 574–642.
|
| ○ |
Govaerts, R. & Frodin. D. G., 1998. World Checklist and Bibliography of Fagales. Kew: Royal Botanic Gardens. Kew. 408 pp.
|
| ○ |
Harris, R., 1979. A glossary of surface sculpturing. Occasional Papers in Entomology, 28: 1–31.
|
| ○ |
Hartig, T., 1840. Ueber die Familie der Gallwespen. III. Zeitschrift für Entomologie (Germar), 2: 176–209.
|
| ○ |
Kappelle, M., 1996. Los Bosques de Roble (Quercus) de la Cordillera de Talamanca, Costa Rica: biodiversidad, ecología, conservación y desarrollo. Instituto Nacional de Biodiversidad y Universidad de Amsterdam. Heredia. 336 pp.
|
| ○ |
Kinsey, A. C., 1923. The gall wasp genus Neuroterus (Hymenoptera). Indiana University Studies, 58: 1–150.
|
| ○ |
Kinsey, A. C., 1938. New Mexican gall wasps (Hymenoptera, Cynipidae) IV. Proceedings of the Indiana Academy of Sciences, 47: 261–280.
|
| ○ |
Liljeblad, J., Ronquist, F., Nieves-Aldrey, J. L, Fontal-Cazalla, F., Ros-Farre, P., Gaitros, D., & Pujade-Villar, J., 2008. A fully web-illustrated morphological phylogenetic study of relationships among oak gall wasps and their closest relatives (Hymenoptera: Cynipidae). Zootaxa, 1796: 1–73.
|
| ○ |
Medianero, E. & Nieves-Aldrey, J. L., 2011. Primer estudio de las avispas de las agallas de la República de Panamá, incluyendo una lista actualizada de los cinípidos neotropicales (Hymenoptera, Cynipidae). Boletín de la S.E.A., 48: 89–104.
|
| ○ |
Medianero, E. & Nieves-Aldrey, J. L., 2013. Barucynips panamensis, a new genus and species of oak gallwasps (Hymenoptera: Cynipidae: Cynipini) from Panama, and description of a new species of Coffeikokkos. Zookeys, 277: 25–46. http://dx.doi.org/10.3897/zookeys.277.3942
|
| ○ |
Medianero, E. & Nieves-Aldrey, J. L., 2014 Callirhytis cameroni: a new species of oak gall wasp (Hymenoptera: Cynipidae: Cynipini) from Panama. Florida Entomologist, 97(4): 1710–1717. http://dx.doi.org/10.1653/024.097.0446
|
| ○ |
Melika, G. & Abrahamson, W. G., 1997. Descriptions of four new species of cynipid gall wasps of the genus Neuroterus (Hymenoptera: Cynipidae) with redescriptions of some known species from the eastern United States. Proceedings of the Entomological Society of Washington, 99: 560–573.
|
| ○ |
Melika, G., & Abrahamson W. G., 2002. Review of the world genera of oak cynipid wasps (Hymenoptera:Cynipidae, Cynipini). In: G. Melika & C. Thuróczy (eds.). Parasitic Wasps: Evolution, Systematics, Biodiversity and Biological Control. Agroinform. Budapest: 150–190.
|
| ○ |
Melika, G., Pujade-Villar, J., Abe, Y., Tang, C. T., Nicholls, J., Wachi, N., Ide, T., Yang, M. M., Pénzes, Z. S., Csóka, G. Y. & Stone, G. N. 2010. Palaearctic oak gallwasps galling oaks (Quercus) in the section Cerris: re-appraisal of generic limits, with descriptions of new genera and species (Hymenoptera: Cynipidae: Cynipini). Zootaxa, 2470: 1–79.
|
| ○ |
Nieves-Aldrey, J. L., 2001. Hymenoptera, Cynipidae. In: M. A. Ramos, J. Alba-Tercedor, X. Bellés-i-Ros, J. Gosálbez-i-Noguera, A. Guerra-Sierra, E. Macpherson-Mayol, F. Martín-Piera, J. Serrano-Marino & J. Templado-González (eds.). Fauna Ibérica. Vol. 16. Museo Nacional de Ciencias Naturales CSIC. Madrid: 1–636.
|
| ○ |
Nieves-Aldrey, J. L., Liljeblad, J., Hernández-Nieves, M., Grez, A., & Nylander, J. A. A., 2009. Revision and phylogenetics of the genus Paraulax Kieffer (Hymenoptera, Cynipidae) with biological notes and description of a new tribe, a new genus, and five new species.
Zootaxa, 2200: 1–40.
|
| ○ |
Nieves-Aldrey, J. L. & Medianero, E., 2010. Agastoroxenia panamensis, a new genus and species of inquiline oak gallwasps (Hymenoptera: Cynipidae: Synergini) of the Neotropics. Annals of the Entomological Society of America, 103 (4): 492–499.
|
| ○ |
Nieves-Aldrey, J. L., Rodríguez, P. A. & Medianero, E., 2013. Description of a new species of Diastrophus (Hymenoptera: Cynipidae: Aylacini) from Colombia: the first herb gall wasp native to the Neotropical Region. Annals of the Entomological Society of America, 106(6): 719–728. http://dx.doi.org/10.1603/AN13033
|
| ○ |
Nieves-Aldrey, J. L. & San Blas, G., 2015. Revision of the Neotropical genus Eschatocerus Mayr (Hymenoptera, Cynipidae, Eschatocerini) with biological notes and the first description of the terminal larva. Zootaxa, 4012: 135–155. http://dx.doi.org/10.11646/zootaxa.4012.1.7
|
| ○ |
Pujade-Villar, J., 2008. Description of Odontocynips hansoni n. sp., from Costa Rica (Hymenoptera. Cynipidae). Dugesiana, 15: 79–85.
|
| ○ |
Pujade-Villar, J., Cibrián-Tovar, D., Barrera-Ruíz, U.M. & Melika, G., 2015. First record of Neuroterus galls on twigs in Mexico with description of two new species (Hym.: Cynipidae). Butlletí de la Institució Catalana d’Història Natural, 78: 3–8. 2014
|
| ○ |
Pujade-Villar, J., García-Martiñón, R. D., Equihua-Martínez, A., Estrada-Venegas, E. & Ferrer-Suay, M., 2016. Neuroterus fusifex Pujade-Villar and Ferrey-Suay n. sp. (Hymenoptera: Cynipidae): first record of galls on catkins in Mexico. Folia Entomológica Mexicana, 2(3): 75–83.
|
| ○ |
Pujade-Villar, J., Hanson, P., Medina, C. A., Torres, M. & Melika, G., 2012b. A new genus of oak gallwasps, Zapatella Pujade-Villar & Melika, gen. n., with a description of two new species from the Neotropics (Hymenoptera, Cynipidae, Cynipini). ZooKeys, 210: 75–104. https://doi.org/10.3897/zookeys.210.3014
|
| ○ |
Pujade-Villar, J., Hanson, P., Melika, G., 2012a. A new genus of oak gallwasp, Coffeikokkos Pujade-Villar & Melika, gen. n., with a description of a new species from Costa Rica (Hymenoptera, Cynipidae). ZooKeys, 168: 19–29. http://dx.doi.org/10.3897/zookeys.168.2030
|
| ○ |
Pujade-Villar, J. & Rodríguez, P. A., 2015. Primera cita del género Melikaiella (Hym. Cinipidae) para Colombia y descripción de una nueva especie. Orsis, 29: 193–404.
|
| ○ |
Ronquist, F., 1995. Phylogeny and early evolution of the Cynipoidea (Hymenoptera). Systematic Entomology, 20: 309–335. http://dx.doi.org/10.1111/j.1365-3113.1995.tb00099.x
|
| ○ |
Ronquist, F., Nieves-Aldrey, J. L., Buffington, M. L., Liu, Z., Liljeblad, J. & Nylander, J. A. A., 2015. Phylogeny, Evolution and Classification of Gall Wasps: The Plot Thickens. PLoS ONE 10(5): e0123301. http://dx.doi.org/10.1371/journal.pone.0123301
|
| ○ |
Ronquist, F. & Nordlander, G., 1989. Skeletal morphology of an archaic cynipoid, Ibalia rufipes (Hymenoptera, Ibaliidae). Entomol. Scandinavica Supplementum, 33: 1–60.
|
| ○ |
Stone, G. N., Hernández-López, A., Nicholls, J. A., Di Pierro, E., Pujade-Villar, J., Melika, G., & Cook, J. M., 2009. Extreme host plant conservatism during at least 20 million years of host plant pursuit by oak gallwasps. Evolution, 63(4): 854–869. http://dx.doi.org/10.1111/j.1558-5646.2008.00604.x
|
| ○ |
Tang, C. T., Melika, G., Nicholls, J. A., Yang, M.-M., & Stone, G. N., 2011. A new genus of oak gallwasps, Cycloneuroterus Melika & Tang, with the description of five new species from Taiwan (Hymenoptera; Cynipidae: Cynipini). Zootaxa. 3630(3): 534–548.
|
| ○ |
Tang, C. T., Sinclair, F., Hearn, J., Yang, M. M., Stone, G. N., Nicholls, J. A. & Melika, G., 2016. Eight new species of Cycloneuroterus Melika & Tang gallwasps from Taiwan and mainland China (Hymenoptera: Cynipidae: Cynipini). Zootaxa, 4088(4), 451–488. http://dx.doi.org/10.11646/zootaxa.4088.4.1
|
| ○ |
Valencia-A, S., 2004. Diversidad del género Quercus (Fagaceae) en México. Boletín de la Sociedad Botánica de México, 75: 33–53.
|
| ○ |
Van Noort, S., Stone, G. N., Whitehead, V. B. & Nieves-Aldrey, J. L., 2007. Biology of Rhoophilus loewi (Hymenoptera: Cynipoidea: Cynipidae), with implications for the evolution of inquilinism in gall wasps. Biological Journal of the Linnaean Society, 90: 153–172. http://dx.doi.org/10.1111/j.1095-8312.2007.00719.x
|
| ○ |
Weld, L. H., 1952. Cynipoidea (Hym.) 1905–1950. Privately published, Ann Arbor, Michigan, 351 pp.
|
Fig. 1.—Neuroterus elvisi sp. n., adult (SEM): (A) Head, anterior view. (B) Head posterior view. (C) Head, dorsal view. (D) Head, lateral view. (E) Female antenna. (F) Male antenna.