TAXONOMIC REMARKS ON BARBUS MOULOUYENSIS PELLEGRIN, 1924 (ACTINOPTERYGII, CYPRINIDAE) WITH THE DESCRIPTION OF A NEW SPECIES OF LUCIOBARBUS HECKEL, 1843 FROM MOROCCO
Ignacio Doadrio*, Miriam Casal-López & Silvia Perea
Biodiversity and Evolutionary Group, Museo Nacional de Ciencias Naturales, CSIC. C/José Gutiérrez Abascal, 2, 28006 Madrid, Spain
*Corresponding author: doadrio@mncn.csic.es
| |
ABSTRACT
The taxonomy of Barbus Cuvier and Cloquet, 1816 has been reviewed in the last years and as consequence some species traditionally included in genus Barbus sensu lato have been assigned to different genera. In North Africa the species of the former genus Barbus have been included in the genera Luciobarbus Heckel, 1843, Carasobarbus Karaman, 1971 and Enteromius Cope, 1867. We studied populations of the former genus Barbus of the Moulouya river basin in Morocco through molecular, morphometric, and osteological data. Our data clearly showed that populations from Moulouya river basin described originally as Barbus moulouyensis Pellegrin, 1924 belong to the genus Carasobarbus and not to Luciobarbus. Moreover, populations of the genus Luciobarbus exist in the Moulouya river basin and could not be assigned to any previously described species. Consequently, we describe a new Luciobarbus species from the Moulouya river basin.
http://urn:lsid:zoobank.org:pub:F714D4AD-9591-4A19-83D0-EBAF134A8BC6
Key words: North Africa;
Luciobarbus;
Systematics;
mtDNA;
morphology.
|
| |
RESUMEN
Consideraciones taxonómicas sobre Barbus moulouyensis Pellegrin, 1924 (Actinopterygii, Cyprinidae) con la descripción de una especie nueva de Luciobarbus Heckel, 1843 de Marruecos
En los últimos años ha sido revisada la taxonomia de Barbus Cuvier and Cloquet, 1816 y como consecuencia de esta revisión algunas especies incluidas tradicionalmente en el género Barbus sensu lato han sido asignadas a otros géneros. En el Norte de África las especies del antiguo género Barbus han sido adscritas a los géneros Luciobarbus Heckel, 1843, Carasobarbus Karaman, 1971 y Enteromius Cope, 1867. Nosotros estudiamos las especies del antiguo género Barbus en la cuenca del río Moulouya en Marruecos a través de datos moleculares, morfométricos y osteológicos. Nuestros datos muestran claramente que las poblaciones de la cuenca del río Moulouya descritas como Barbus moulouyensis Pellegrin, 1924 pertenecen al género Carasobarbus y no al género Luciobarbus. Sin embargo, poblaciones del género Luciobarbus existen en la cuenca del río Moulouya y no pudieron ser asignadas a ninguna de las especies previamente descritas. En consecuencia nosotros describimos una nueva especie de la cuenca del río Moulouya.
Palabras clave: África del Norte;
Sistemática;
Morfología;
mtDNA;
Luciobarbus.
|
IntroductionTOP
The freshwater fish fauna of North Africa is mainly characterized by the presence of barbel species inhabiting different habitats
that drain to Mediterranean and Atlantic Sea or to endorheic lagoons (Doadrio, 1994). Those species can be grouped by their different levels of ploidy in diploids, tetraploids and hexaploids species. All of
them were traditionally assigned to the genus Barbus Cuvier and Cloquet, 1816 (Pellegrin, 1921; Estève, 1947; Almaça, 1966, 1968, 1970).
Posterior phylogenetic studies based on morphological and molecular traits have placed the diploids, tetraploids and hexaploids
barbel species from North Africa in different genera (Machordom & Doadrio, 2001a; Levin et al., 2012; Borkenhagen & Krupp, 2013; Casal-López et al., 2015; Yang et al., 2015; Beshera et al., 2016). Thus, diploid species were placed in the genus Enteromius Cope, 1867, tetraploids in Luciobarbus Heckel, 1843 and all hexaploid species in Carasobarbus Karaman, 1971, except “Barbus” reinii Günther, 1874 that remains without a clear generic assignation.
In Morocco, the genera Luciobarbus, Carasobarbus and the enigmatic “Barbus” reinii are only present (Beshera et al., 2016). The genus Luciobarbus is composed of two different group of species: reophilic species of small body size and limnetic species of medium-large size (Doadrio, 1990; Doadrio et al., 2016). The genus Carasobarbus in Morocco is constituted by C. fritschii (Günther, 1874), a cosmopolitan species of small body size, and C. harterti (Günther, 1901), a species of large body size that inhabit only the large basins of the Atlantic slope from Morocco (Borkenhagen & Krupp, 2013).
The Moulouya River with 530 km in length and with a basin surface of 54,500 Km2 represents the largest river of Morocco. Its sources are placed in the Atlas Mountains and flows into Mediterranean Sea near
the Algerian border. In Moulouya Basin an endemic species, Barbus moulouyensis Pellegrin, 1924, was described on the basis of the morphological traits of one single individual of Carasobarbus. However some traits of “Barbus” moulouyensis as the morphology of the scales or the last dorsal fin ray denticulated were typical characters of genus Luciobarbus not of Carasobarbus (Doadrio, 1990; Borkenhagen & Krupp, 2013). Thus, the presence of traits of Carasobarbus and Luciobarbus placed “Barbus” moulouyensis in an uncertain taxonomic position.
Laterly, two varieties of “Barbus” moulouyensis were described, also with one single specimen for each variety: “Barbus” moulouyensis var. grandisquamata Pellegrin, 1930 from Tensift Basin and “Barbus” moulouyensis var. bouramensis Pellegrin, 1939 from Oum er Rbia Basin, both on the Atlantic slope of Morocco. Therefore, none of these varieties were found in the Moulouya
River Basin. Currently, the fish fauna from Tensift and Oum er Rbia basins is considered to be comprised by the following
barbel species: Luciobarbus magniatlantis (Pellegrin, 1919); Luciobarbus zayanensis Doadrio, Casal-López and Yahyaoui, 2016; Luciobarbus ksibii (Boulanger, 1905); Carasobarbus fritschii (Günther, 1874) and Carasobarbus harterti (Günther, 1901) (Borkenhagen & Krupp, 2013; Geiger et al., 2014; Doadrio et al., 2016). On the basis of three individuals, one of each variety, the differences found between typical “Barbus” moulouyensis from Moulouya Basin and its varieties “Barbus” moulouyensis var. grandisquamata and “Barbus” moulouyensis var. bouramensis are referred to small differences in the number of scales on the lateral line, barbels size and pectoral fin length (Pellegrin,
1930, 1939).
The first phylogenetic work on tetraploid barbel species from North Africa, based on molecular markers, named the specimens
studied from Moulouya Basin as Barbus cf. moulouyensis, indicating the uncertainty in attributing the specimens to the species described by Pellegrin (1924) as Barbus moulouyensis (Machordom et al., 1998). Subsequently, molecular works removed the expression “cf.” referring to the same individuals, or individuals of the same
population. In this direction Barbus cf. moulouyensis is referred in posterior molecular works as Barbus moulouyensis and currently as Luciobarbus moulouyensis (Machordom & Doadrio, 2001b; Tsigenopoulos et al., 2003; Berrebi et al., 2014, Geiger et al., 2014; Yang et al., 2015). Thus, the uncertainty on the generic assignation of “Barbus” moulouyensis and on the correct assignation of the individuals from Moulouya Basin, in phylogenetic studies, remains until present.
We hypothetized that “Barbus” moulouyensis should be assigned to genus Carasobarbus and that individuals present in former phylogenetic studies, from Moulouya Basin belong to one undescribed species of the
genus Luciobarbus.
To test this hypothesis we analyzed the largest number of individuals from Moulouya Basin studied so far through molecular,
morphometric and osteological traits.
Material and MethodsTOP
Our study of “Barbus” moulouyensis was based on populations of different localities along Moulouya River and its tributaries including the Terra
Typica of “Barbus” moulouyensis in the Za River (Oued el Haï) in Guefait. The individuals collected in Moulouya Basin were identified as hexaploid Carasobarbus or tetraploid Luciobarbus species following the morphological traits established in taxonomic works (Doadrio, 1990; Borkenhagen & Krupp, 2013; Doadrio et al., 2016) and lately confirmed by the sequencing of the mitochondrial cytochrome b gene. We studied also Holotypes of Barbus moulouyensis Pellegrin, 1924, Barbus moulouyensis var. bouramensis Pellegrin, 1939 and the possible Holotype of Barbus moulouyensis var. grandisquamata Pellegrin, 1930, kept in the Museum of Comparative Zoology of Harvard. For comparative purpose we included the limnetic Luciobarbus species geographically closer to Moulouya Basin.
The material studied comprised the following specimens and localities: Luciobarbus specimens from Moulouya Basin: 11 specimens (3 females and 8 males) from Moulouya River in Ghafoula, Morocco (voucher numbers
MNCN_ICTIO 290.951-290.961); 26 specimens (2 females, 23 males and 1 undet.) from Moulouya River in Ksabi, Morocco (voucher
numbers MNCN: MNCN_ICTIO 290.864-290.878, 290.880-290.885, 290.887-290.991); 12 specimens (3 females, 8 males and 1 undet.)
from Melloulou River in Guercif, Morocco (voucher numbers: MNCN_ICTIO 290.995-290.997, 290.998-291.006); 27 specimens (11
females and 16 males) from Zobzite River in Berkine, Morocco (voucher numbers: MNCN_ICTIO 290.910-290.936); 8 specimens (2
females and 6 males) from Za River in Guefait, Morocco (Type Locality of “Barbus” moulouyensis) (voucher numbers: MNCN_ICTIO 71.606-71.611, 71.613-71.614). Carasobarbus cf. fritschii specimens from Moulouya Basin: 6 males from Moulouya River in Ghafoula, Morocco (voucher numbers: MNCN_ICTIO 290.899-290.904);
2 males from Moulouya River in Ksabi, Morocco (voucher numbers: MNCN_ICTIO 290.897-290.898); 5 specimens (4 females and 1
male) from Melloulou River, in Guercif, Morocco (voucher numbers: MNCN_ICTIO 290.989-290.992, 290.994); 3 specimens from El
Barred River in Asrire, Morocco (voucher numbers: MNCN_ICTIO 290.907-290.909). Luciobarbus rifensis: 47 specimens (17 females and 30 males) from Laou River (Laou Basin) in Derdara (voucher numbers: MNCN_ICTIO 290.639-290.652,
290.655, 290.657-663, 290.665-667) and Beni Fertan (Voucher numbers: MNCN_ICTIO 284.939-940, 284.942-945, 284.947-284.948,
284.950-951, 284.953-964), Morocco. Luciobarbus maghrebensis: 55 specimens (11 males and 44 females) from Ifrane River (Sebou Basin) in Ouad Ifrane (voucher numbers: MNCN_ICTIO 279.711-729,
290.731, 279.733-744) and Tizguit River (Sebou Basin) in Ifrane (Voucher numbers: MNCN_ICTIO 71.675-71.697), Morocco. Luciobarbus setivimensis. 15 specimens from Soummam River (Soummam Basin) in Takretz, Algeria (voucher numbers: MNCN_ICTIO 106.148-106.162). Luciobarbus ksibi: 28 specimens (20 males and 8 females) from Derna River (Oum er Rbia Basin) in Bounoval, Morocco (voucher numbers: MNCN 291.122-291.149).
Barbus moulouyensis: Holotype Oued el Haï (Za River), in Guefait, Morocco (MNHN 1924-0167). Barbus moulouyensis var. bouramensis: Holotype Aïn Bouram in El ksiba- Tezghrit, Morocco (MNHN 1939-0121). Barbus moulouyensis var. grandisquamata. Holotype? Oued Tensift near of Marrakesh, Morocco (MCZ 32741) (Fig. 1; Table 1).
|
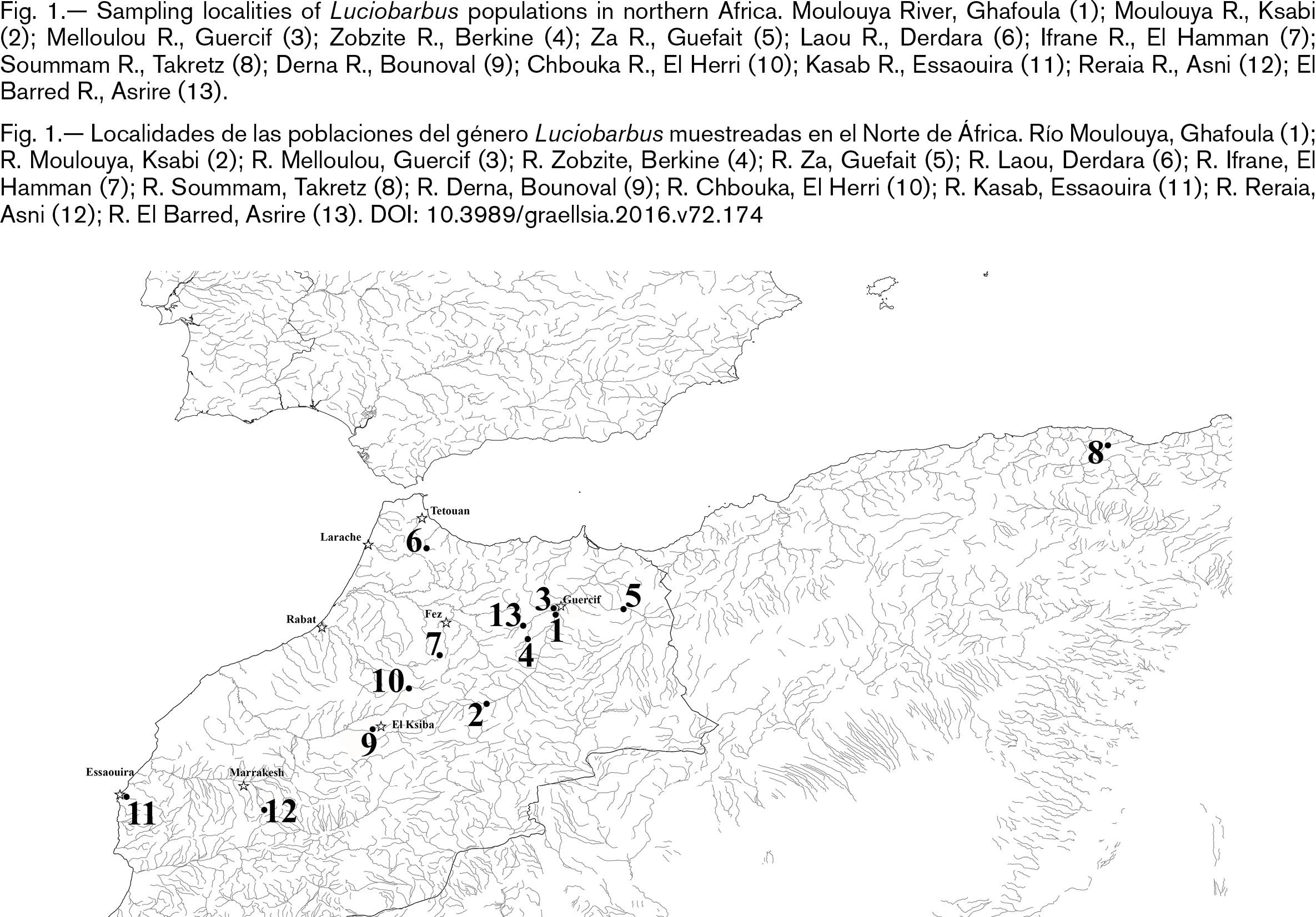 Fig. 1.— Sampling localities of Luciobarbus populations in northern Africa. Moulouya River, Ghafoula (1); Moulouya R., Ksabi (2); Melloulou R., Guercif (3); Zobzite R., Berkine (4); Za R., Guefait (5); Laou R., Derdara (6); Ifrane R., El Hamman (7); Soummam R., Takretz (8); Derna R., Bounoval (9); Chbouka R., El Herri (10); Kasab R., Essaouira (11); Reraia R., Asni (12); El Barred R., Asrire (13). Fig. 1.— Sampling localities of Luciobarbus populations in northern Africa. Moulouya River, Ghafoula (1); Moulouya R., Ksabi (2); Melloulou R., Guercif (3); Zobzite R., Berkine (4); Za R., Guefait (5); Laou R., Derdara (6); Ifrane R., El Hamman (7); Soummam R., Takretz (8); Derna R., Bounoval (9); Chbouka R., El Herri (10); Kasab R., Essaouira (11); Reraia R., Asni (12); El Barred R., Asrire (13).
Fig. 1.— Localidades de las poblaciones del género Luciobarbus muestreadas en el Norte de África. Río Moulouya, Ghafoula (1); R. Moulouya, Ksabi (2); R. Melloulou, Guercif (3); R. Zobzite, Berkine (4); R. Za, Guefait (5); R. Laou, Derdara (6); R. Ifrane, El Hamman (7); R. Soummam, Takretz (8); R. Derna, Bounoval (9); R. Chbouka, El Herri (10); R. Kasab, Essaouira (11); R. Reraia, Asni (12); R. El Barred, Asrire (13).
|
|
Table 1.— Sampling localities for Luciobarbus and Carasobarbus and GenBank Accession numbers.
Tabla 1.— Localidades de muestreo para Luciobarbus y Carasobarbus y números de acceso de GenBank.
| Population assignment/species |
Locality |
No. Individuals studied Morphology/molecular |
Code in phylogenetic tree |
GenBank Accession Numbers |
Number in Map |
| Moulouya population |
Moulouya R. Ghafoula/Moulouya Basin |
11/3 |
M4,M8-M9 |
KX681705, KX681704, KX681706 |
1 |
| Moulouya population |
Moulouya R. Ksabi/Moulouya Basin |
26/- |
|
|
2 |
| Moulouya population |
Melloulou R. Guercif/Moulouya Basin |
12/3 |
M5-M7 |
KX681701-681703 |
3 |
| Moulouya population |
Zobzite R. Berkine/Moulouya Basin |
27/3 |
M1-M3 |
KX681698-KX681700 |
4 |
| Moulouya population |
Za R. Guefait/ Moulouya Basin |
8/- |
|
|
5 |
| Luciobarbus rifensis |
Laou R. Derdara/ Laou Basin |
47/4 |
R1-R4 |
KT003027-KT003930 |
6 |
| Luciobarbus maghrebensis |
Ifrane R. El Hamman/Sebou Basin |
55/4 |
Ma1-Ma4 |
KT003941,KT003943-KT003945 |
7 |
| Luciobarbus setivimensis |
Soummam R. Takretz/ Soummam Basin |
15/3 |
ST1-3 |
AY004748; KX681686-KX681687 |
8 |
| Luciobarbus ksibi |
Derna R. Bounoval/Oum er Rbia Basin |
29/- |
|
|
9 |
| Luciobarbus ksibi |
Chbouka R. El Herri/Oum er Rbia Basin |
-/2 |
K5, K6 |
KU257529-30 |
10 |
| Lucibarbus ksibi |
Kasab R. Essaouira/ Kasab Basin |
-/2 |
K1, K2 |
KU257523-24 |
11 |
| Luciobarbus ksibi |
Reraia R. Asni/Tensift Basin |
-/2 |
K3,K4 |
KU257538-39 |
12 |
Carasobarbus cf. fritschii
from Moulouya Basin
|
Moulouya R. Ghafoula
Moulouya R. Ksabi
Melloulou R. Guercif
El Barred R. Asrire |
6/-
2/-
6/-
3/- |
|
|
1
2
3
13 |
| Luciobarbus guercifensis |
Moulouya R. Ghafoula/ Moulouya Basin
Melloulou R. Guercif/ Moulouya Basin |
-/7
-/3 |
Ge1-Ge4, Ge6,Ge8-9
Ge5,Ge7,Ge10 |
KU257526, KX681697, KX681695, KX681696 KU257527, KX681693 KU257525
KU257528; KX681691-KX681692 |
1
3 |
| L. bocagei |
Tajo R./Tajo Basin
Alberche R./Tajo Basin
Jerte R./ Tajo Basin |
-/1
-/1
-/1 |
B1
B3
B2 |
AF112125
AF334054
AF334064 |
|
| L.comizo |
Guadiana R./ Guadiana Basin
Tiétar R./ Tajo Basin
Tajo R. /Tajo Basin |
-/1
-/1
-/1 |
C2
C3
C1 |
AF334047
AF334042
AY004735 |
|
| L. graellsii |
Irati R./Ebro Basin
Cadagua R./ Nervión Basin
Mesa R./ Ebro Basin |
-/1
-/1
-/1 |
Gr2
Gr3
Gr1 |
AF334088
JF798258
AF334089 |
|
| L. guiraonis |
Turia R./Turia Basin
Júcar R./Júcar Basin
Palancia R/ Palancia Basin |
-/1
-/1
-/1 |
Gu2
Gu3
Gu1 |
AF334094
AF334093
AF334097 |
|
| L. microcephalus |
Guadiana R./ Guadiana Basin
Estena R./ Guadiana Basin |
-/2
-/1
-/1 |
Mc1
Mc2
Mc3 |
AF334085
AF334084
AF045971 |
|
| L. sclateri |
Segura R./Segura Basin
Guadalquivir R./ Guadalquivir Basin. Guadiato R./ Guadalquivir Basin |
-/1
-/1
-/1 |
SC1
SC2
SC3 |
AF334071
AF334070
AF334069 |
|
Twenty-four morphometric measurements were taken with digital callipers (0.01 mm), and ten meristic variables were counted
with a stereoscopic microscope. The following acronyms were used for morphometric and meristic characters: A, number of anal
fin rays; AFH, anal fin height; AFL, anal fin length; APL, anal peduncle length; BL1, first barbel length; BL2, second barbel
length; BD, body depth; BLD, body least depth; C, central caudal fin rays; CFL, caudal fin length; CPL, caudal peduncle length;
D, dorsal fin rays, DFL dorsal fin length; DFH dorsal fin height; ED, eye diameter; GR, gill rakers (number); HL, head length;
IOW, interorbital width; LL lateral line scales; P, pectoral fin rays; PFL, pectoral fin length; PrAD, pre-anal distance;
PrDD, pre-dorsal distance; PrOL, pre-orbital length; PrPD, pre-pectoral distance; PrVD, pre-ventral distance; PsOL, postorbital
length; PVL, pectoral-ventral length; RSA, scale rows above lateral line; RSB scale rows below lateral line; SL, standard
length; V, ventral fin rays; VFL, ventral fin length; VE, Number of vertebrae. The number of vertebrae was obtained by counting
on X-ray images of specimens from all sampled populations. Osteological characteristics were investigated through computer
tomography (CT) scan and digital dissection using VGStudio MAX v2.2 (Volume Graphics, http://www.volumegraphics.com).
After constructing the measurement matrix, Burnaby’s method was used to correct size effect. The Burnaby method removes the
effects of a within population size-factor from between-group morphometric analyses through an orthogonal projection procedure
(Burnaby, 1966; Röhlf & Bookstein, 1987). All analyses were conducted with the corrected matrix. Morphometric and meristic characters were analysed independently.
To identify the variables that contributed most to the variation among populations, two principal component analyses (PCA)
were performed using the covariance matrix for morphometric characters. Statistical analyses were carried out using PAST software
(Hammer et al., 2001).
MOLECULAR ANALYSES
For the molecular approach we analyzed all populations morphologically studied of the genus Luciobarbus. Also, all the other Moroccan species of genus Luciobarbus were added. The species Aulopyge hueguelli Heckel, 1843 and Barbus meridionalis Risso, 1827 were selected as outgroups based on previous phylogenetic analyses (Zardoya & Doadrio, 1999). Total genomic DNA was extracted from fin-clip tissue using the commercial kit Biosprint 15 for tissue and blood (Qiagen). For each specimen, the complete region (1140bp) of the mitochondrial cytochrome b (cytb) was amplified. Primers and protocols used for PCR for cytb followed Machordom & Doadrio (2001b). After checking PCR products on 1% agarose gels, they were purified by ExoSAP-IT™ (USB) and directly sequenced on MACROGEN service using a 3730XL DNA sequencer. All new sequences were deposited in the GenBank database (Accession Numbers: KX681686-KX681687,
KX681691-KX681692, KX681695-KX681706).
PHYLOGENETIC ANALYSES
Phylogenetic analyses were performed using Bayesian inference (BI) implemented in MrBayes v.3.2 (Ronquist et al., 2012). The Akaike Information Criterion (Akaike, 1973) implemented in jModeltest (Posada, 2008) was used to determine the evolutionary model that best fit the data. In this case TIM1+G model was selected (R(a) [AC] =1.0000,
R(b) [AG] = 29.9653, R(c) [AT] = 0.6120, R(d) [CG] = 0.6120, R(e) [CT] =11.9160, R(f) [GT] =1.0000, p-inv =0.1770). BI was
performed using two independent runs of four Markov Montecarlo coupled chains (MCMC) of 5×106 generations each, to estimate the posterior probability distribution. Topologies were sampled every 100 generations, and
majority-rule consensus tree was estimated after discarding the first 10% of generations. Robustness of clades was assessed
using Bayesian posterior probabilities. The average genetic distances among populations were calculated for each gene using
MEGA package v.6.0 (Tamura et al., 2013) according to the uncorrected-p distances.
Results and DiscussionTOP
COMPARISON OF MORPHOLOGY AMONG POPULATIONS
Two-way analysis of variance (ANOVA), testing for sexual dimorphism and differentiation among populations, showed significant
differences (p<0.01) for sexual dimorphism only for the Standard length and Post-orbital distance (PsOL). To deal with the
presence of sexual dimorphism we removed PsOL from posterior analyses. Most morphometric variables showed significant differences
between populations in the two-way ANOVA analysis (Table 2).
Table 2.— Two-way analysis of variance (ANOVA) for sexual dimorphism, population variation, and their interaction. Significant
differences p<0,01 (**). N= 156 males and N= 58 females. Abbreviations are described in the Material and Methods epigraph.
Tabla 2.— Análisis de la varianza (ANOVA) de dos vías para dimorfismo sexual, variación poblacional y su interacción. Diferencias
significativas p<0,01 (**). N= 156 machos y 58 hembras. Las abreviaturas se describen en el epígrafe de Material y Métodos.
| Variables |
Sexual dimorphism (F/significance) |
Population Variation (F/significance) |
Sex/pop Variation (F/significance) |
| SL |
53.58/** |
2.948/ |
10.9/** |
| HL |
0.3594/ |
34.05/** |
0.8619/ |
| PrOL |
0.4139/ |
44.44/** |
2.487/ |
| ED |
3.877/ |
15.55/** |
3.664/** |
| PsOL |
16.95/** |
372.4/** |
3.905/** |
| B1L |
3.171/ |
70.95/** |
1.229/ |
| B2L |
0.0254/ |
91.76/** |
2.076/ |
| PrDD |
0.205/ |
75.83/** |
1.915/ |
| PrPD |
0.5848/ |
21.88/** |
2.85/** |
| PrVD |
0.2496/ |
58.8/** |
4.23/** |
| PrAD |
0.0581/ |
58.32/** |
3.601/** |
| CPL |
2.246/ |
31.16/** |
1.445/ |
| APL |
2.154/ |
22.49/** |
1.77/ |
| PVL |
0.043/ |
23.38/** |
1.371/ |
| BD |
3.927/ |
33.71/** |
1.357/ |
| BLD |
2.752/ |
38.03/** |
1.006/ |
| DFL |
0.1273/ |
7.027/** |
0.9019/ |
| DFH |
0.5455/ |
24.14/** |
1.674/ |
| PFL |
0.3356/ |
6.357/** |
1.204/ |
| VFL |
5.781/ |
24.29/** |
1.109/ |
| AFL |
1.275/ |
5.516/** |
1.72/ |
| AFH |
6.088/ |
20.75/** |
2.143/ |
| CFL |
0.374/ |
5.067/** |
1.145/ |
A first morphometric analyses, through a Principal Component Analyses (PCA) was conducted to place the types of “Barbus” moulouyensis in tetraploid (Luciobarbus) or hexaploid (Carasobarbus) barbel specimens. The PCA clearly divided the barbel specimens from Moulouya Basin into two different groups, one corresponding
to the genus Carasobarbus and the other to the genus Luciobarbus (Fig. 2).
|
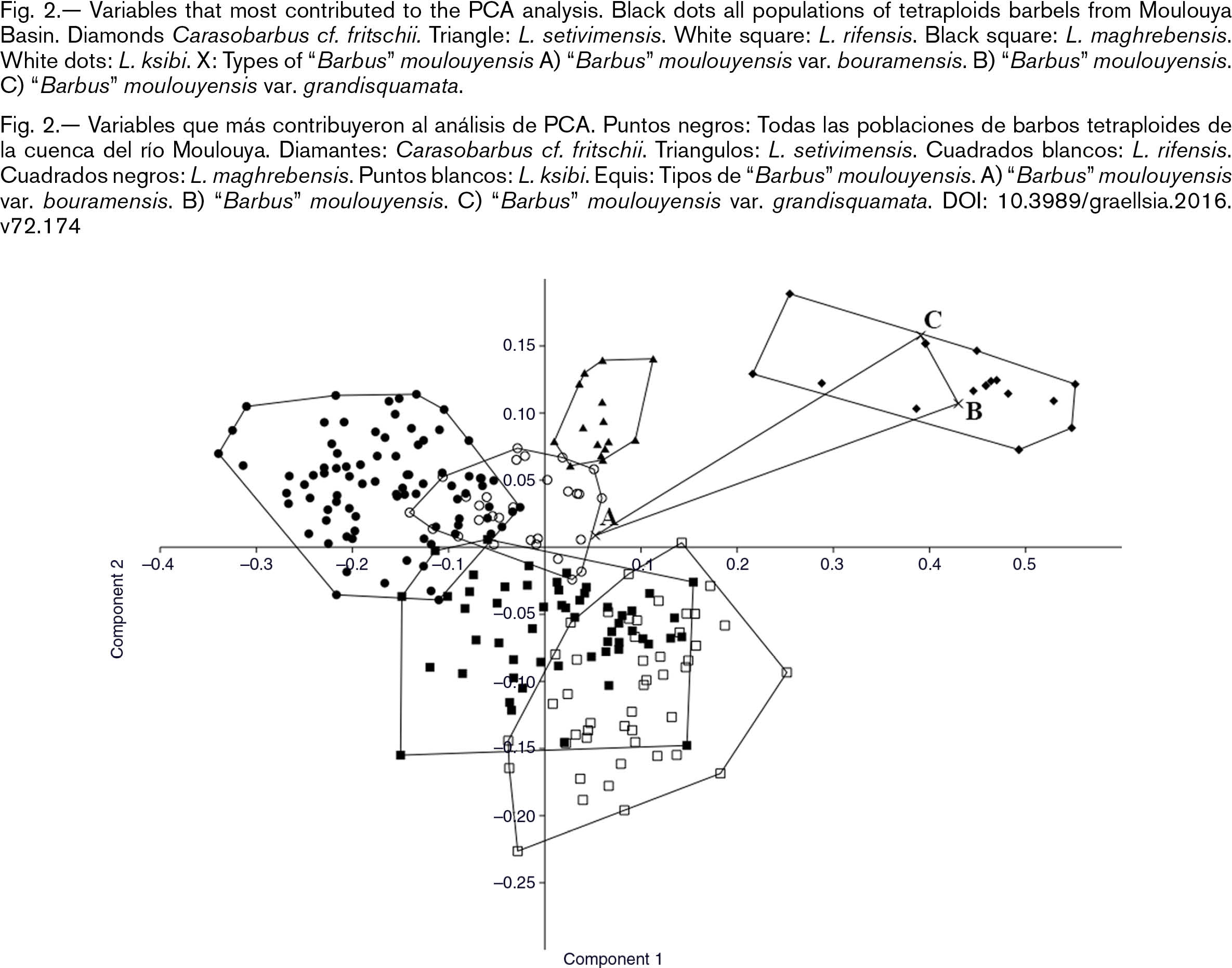 Fig. 2.— Variables that most contributed to the PCA analysis. Black dots all populations of tetraploids barbels from Moulouya Basin. Diamonds Carasobarbus cf. fritschii. Triangle: L. setivimensis. White square: L. rifensis. Black square: L. maghrebensis. White dots: L. ksibi. X: Types of “Barbus” moulouyensis A) “Barbus” moulouyensis var. bouramensis. B) “Barbus” moulouyensis. C) “Barbus” moulouyensis var. grandisquamata. Fig. 2.— Variables that most contributed to the PCA analysis. Black dots all populations of tetraploids barbels from Moulouya Basin. Diamonds Carasobarbus cf. fritschii. Triangle: L. setivimensis. White square: L. rifensis. Black square: L. maghrebensis. White dots: L. ksibi. X: Types of “Barbus” moulouyensis A) “Barbus” moulouyensis var. bouramensis. B) “Barbus” moulouyensis. C) “Barbus” moulouyensis var. grandisquamata.
Fig. 2.— Variables que más contribuyeron al análisis de PCA. Puntos negros: Todas las poblaciones de barbos tetraploides de la cuenca del río Moulouya. Diamantes: Carasobarbus cf. fritschii. Triangulos: L. setivimensis. Cuadrados blancos: L. rifensis. Cuadrados negros: L. maghrebensis. Puntos blancos: L. ksibi. Equis: Tipos de “Barbus” moulouyensis. A) “Barbus” moulouyensis var. bouramensis. B) “Barbus” moulouyensis. C) “Barbus” moulouyensis var. grandisquamata.
|
|
On the basis of morphometric variables the type specimens corresponding to “Barbus” moulouyensis and “Barbus” moulouyensis var. grandisquamata were placed in Carasobarbus group (Fig. 2). In contrast, the type specimen of “Barbus” moulouyensis var. bouramensis from Oum er Rbia Basin was placed along with specimens of Luciobarbus. The variables that more contributed to PCA ordination of Moulouya specimens were barbels and fins size. The barbels were
longer and the fins shorter in Luciobarbus than in Carasobarbus individuals (Table 3).
Table 3.— Eigenvalues and eigenvectors for the first three principal components (PC1-PC3) of 22 morphometric variables for
all populations studied. Acronyms are defined in the Material and Methods section. Variables with the highest eigenvalues
for each PC are in bold.
Tabla 3.— Eigenvalores y eigenvectores para los tres primeros componentes principales (PC1-PC3) de 22 variables morfométricas
para todas las poblaciones estudiadas. Los acrónimos están definidos en la sección de Material y Métodos. Las variables con
los eigenvalores más altos para cada CP están en negrita.
| Variables |
PCI |
PCII |
PCIII |
| Eigenvalue |
0.0302 |
0.0068 |
0.0023 |
| % variance |
61.04 |
13.8 |
4.74 |
| Eigenvectors |
| SL |
0.1111 |
0.0401 |
0.0049 |
| PrDD |
0.0662 |
0.0965 |
0.0875 |
| PrPD |
0.0194 |
0.1596 |
0.1337 |
| PrVD |
0.0851 |
0.1427 |
0.1660 |
| PrAD |
0.1023 |
0.0862 |
0.0792 |
| PVL |
0.1392 |
0.1184 |
0.2225 |
| CPL |
0.1540 |
0.0169 |
0.1309 |
| APL |
0.1443 |
0.1092 |
0.2711 |
| BD |
0.1387 |
0.0621 |
0.1026 |
| BLD |
0.0841 |
0.1171 |
0.2636 |
| HL |
0.0099 |
0.2139 |
0.0025 |
| PrOL |
0.0585 |
0.0989 |
0.0973 |
| ED |
0.0655 |
0.1750 |
0.50343 |
| B1L |
0.6846 |
0.0213 |
0.1304 |
| B2L |
0.5470 |
0.2058 |
0.1866 |
| PFL |
0.1081 |
0.1914 |
0.3899 |
| VFL |
0.0916 |
0.2711 |
0.2885 |
| AFL |
0.1118 |
0.1201 |
0.1650 |
| AFH |
0.0967 |
0.2831 |
01986 |
| DFL |
0.1881 |
0.0720 |
0.0835 |
| DFH |
0.1152 |
0.5012 |
0.1299 |
| CFL |
0.0928 |
0.0598 |
0.2597 |
The adscription of type specimens of “Barbus” moulouyensis to tetraploid (Barbus and Luciobarbus) and not to hexaploid barbels (Labeobarbus or Carasobarbus) was mainly based on the presence of a weakly serrated last single ray in the dorsal fin. Until now a smooth last unbranched
dorsal-fin ray was a diagnostic trait for Carasobarbus (Borkenhagen & Krupp, 2013) but we found in several individuals of Carasobarbus specimens from Moulouya Basin a serrated last unbranched dorsal-fin ray and 8 or 9 branched rays on the dorsal fin (Fig. 3). The denticulations found in individuals of Carasobarbus from Moulouya Basin were even weaker than those found in Luciobarbus specimens of the same localities (Fig. 3). Some grade of introgression of Luciobarbus in those individuals of Carasobarbus with serrated ray could be claimed, but this is difficult to conclude without carrying out a molecular study of the nuclear
genes.
|
 Fig. 3.— Serrated last unbranched dorsal-fin ray shown differences between Carasobarbus and Luciobarbus of similar size. A. Carasobarbus cf. fritschii from Moulouya River, Ghafoula (Moulouya Basin) (MNCN_ICTIO 290.906) 85.4 mm of SL. B Carasobarbus cf. fritschii from El Barred River, Asrire (Moulouya Basin) (MNCN_ICTIO 290.908) 94.1 mm of SL. C. Individual of the Moulouya population from Moulouya River, El ksabi, (Moulouya Basin) (MNCN_ICTIO 290.880) 94.7 mm of Standard Length. D. Luciobarbus sp. from Moulouya River, El ksabi, (Moulouya Basin) (MNCN_ICTIO 290.869) 117.4 mm of SL. Fig. 3.— Serrated last unbranched dorsal-fin ray shown differences between Carasobarbus and Luciobarbus of similar size. A. Carasobarbus cf. fritschii from Moulouya River, Ghafoula (Moulouya Basin) (MNCN_ICTIO 290.906) 85.4 mm of SL. B Carasobarbus cf. fritschii from El Barred River, Asrire (Moulouya Basin) (MNCN_ICTIO 290.908) 94.1 mm of SL. C. Individual of the Moulouya population from Moulouya River, El ksabi, (Moulouya Basin) (MNCN_ICTIO 290.880) 94.7 mm of Standard Length. D. Luciobarbus sp. from Moulouya River, El ksabi, (Moulouya Basin) (MNCN_ICTIO 290.869) 117.4 mm of SL.
Fig. 3.— Último radio no ramificado de la aleta dorsal mostrando las diferencias entre Luciobarbus y Carasobarbus. A. Carasobarbus cf. fritschii del río Moulouya, Ghafoula (Cuenca del Moulouya) (MNCN_ICTIO 290.906) 85.4 mm de longitud estándar. B Carasobarbus cf. fritschii del río El Barred, Asrire (Cuenca del Moulouya) (MNCN_ICTIO 290.908) 94.1 mm de longitud estándar. C. Ejemplar de la población del Moulouya colectado en el río Moulouya, El ksabi, (Cuenca del Moulouya) (MNCN_ICTIO 290.880) 94.7 mm de longitud estándar. D. Luciobarbus sp. del río Moulouya, El ksabi, (Cuenca del Moulouya) (MNCN_ICTIO 290.869) 117.4 mm de LS.
|
|
The three type specimens of “Barbus” moulouyensis had less number of scales than all Luciobarbus samples studied and were in the rank of the samples of Carasobarbus, with the exception of the type of “Barbus” moulouyensis var. bouramensis which had the lowest number of scales on the lateral line of all the barbels (Carasobarbus and Luciobarbus) studied (Fig. 4).
|
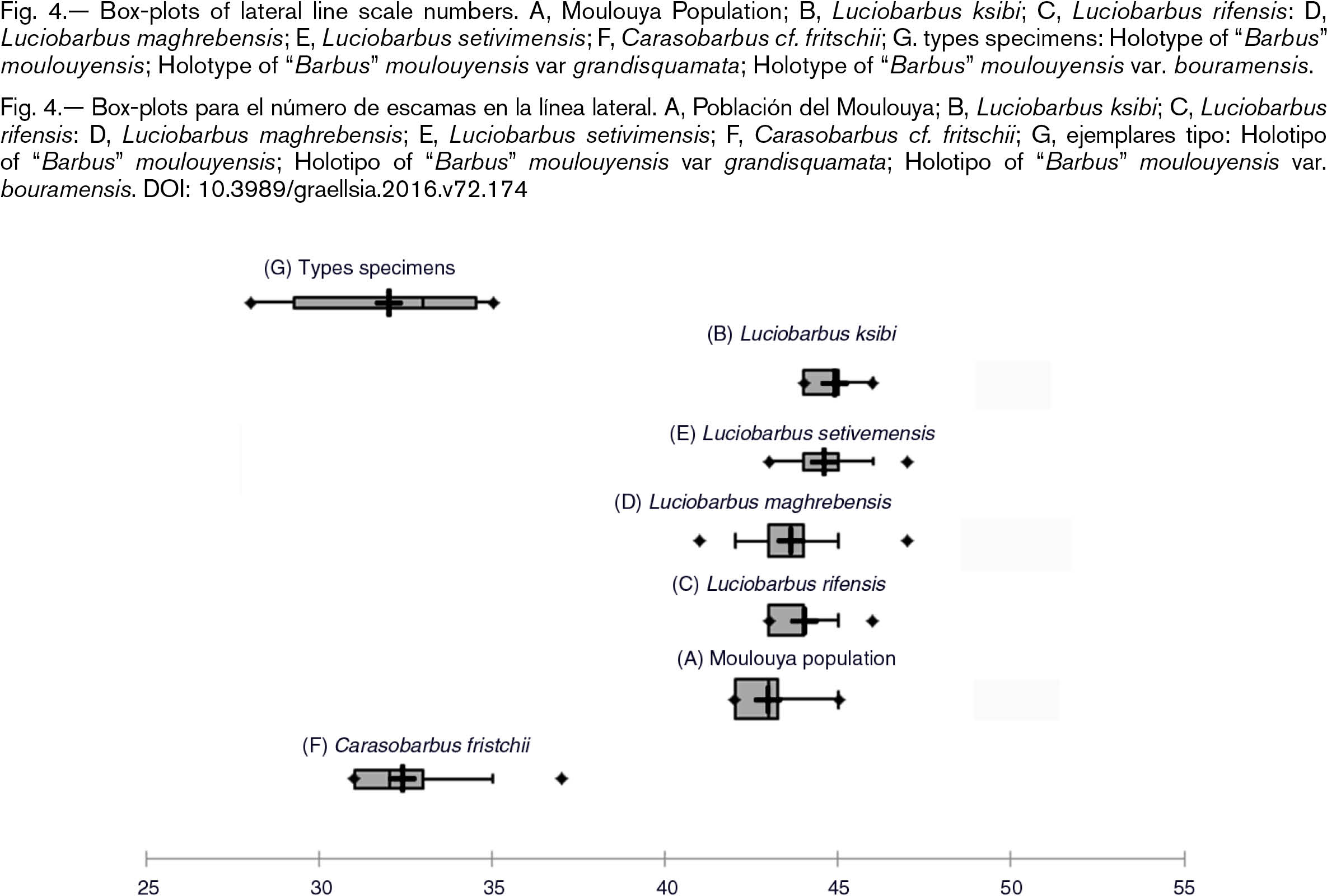 Fig. 4.— Box-plots of lateral line scale numbers. A, Moulouya Population; B, Luciobarbus ksibi; C, Luciobarbus rifensis: D, Luciobarbus maghrebensis; E, Luciobarbus setivimensis; F, Carasobarbus cf. fritschii; G. types specimens: Holotype of “Barbus” moulouyensis; Holotype of “Barbus” moulouyensis var grandisquamata; Holotype of “Barbus” moulouyensis var. bouramensis. Fig. 4.— Box-plots of lateral line scale numbers. A, Moulouya Population; B, Luciobarbus ksibi; C, Luciobarbus rifensis: D, Luciobarbus maghrebensis; E, Luciobarbus setivimensis; F, Carasobarbus cf. fritschii; G. types specimens: Holotype of “Barbus” moulouyensis; Holotype of “Barbus” moulouyensis var grandisquamata; Holotype of “Barbus” moulouyensis var. bouramensis.
Fig. 4.— Box-plots para el número de escamas en la línea lateral. A, Población del Moulouya; B, Luciobarbus ksibi; C, Luciobarbus rifensis: D, Luciobarbus maghrebensis; E, Luciobarbus setivimensis; F, Carasobarbus cf. fritschii; G, ejemplares tipo: Holotipo of “Barbus” moulouyensis; Holotipo of “Barbus” moulouyensis var grandisquamata; Holotipo of “Barbus” moulouyensis var. bouramensis.
|
|
In conclusion, the morphometric traits and scales counts as well as the morphology of the last unbranched dorsal-fin ray placed
unequivocally the types of “Barbus” moulouyensis from Moulouya Basin and “Barbus” moulouyensis var. grandisquamata from Tensift Basin in the genus Carasobarbus. The ascription to Carasobarbus fritschii, a species widely distributed in Morocco (Doadrio, 1994) or to another different species, which could be named Carasobarbus moulouyensis (Pellegrin, 1924), is not the focus of this work and should be addressed taking into account populations of all the distribution range of
Carasobarbus.
Different is the case of “Barbus” moulouyensis var. bouramensis that was described on the basis of one individual from Aïn Bouram, (Bouram Spring), 300 meters from the Ksiba to Taghzirt trail, Morocco. Bouram Spring could not be found despite of our sampling efforts from Ksiba to Taghzirt trail. The only river with fishes was Derna River (Oum er Rbia Basin), which flows through the Bouhzam Mountains. In this river we only sampled Luciobarbus ksibi, Carasobarbus fritschii, Luciobarbus zayanensis and Pterocapoeta maroccana Günther, 1902. The morphometric data placed “Barbus” moulouyensis var. bouramensis within Luciobarbus populations but the number of scales on the lateral line (28) was surprisingly low in comparison to all the species of Luciobarbus that were studied which had more than 40 scales on the lateral line. On the basis of the number of scales and the morphometric variables (Figs. 2 and 4) the Holotype of “Barbus” moulouyensis var. bouramensis could not be assigned to any known population of the genus Luciobarbus and therefore it could be a valid species.
The assignment of the types “Barbus” moulouyensis and “Barbus” moulouyensis var. grandisquamata to genus Carasobarbus resulted in the need of classifying correctly those samples from the Moulouya Basin named in previous molecular works as Barbus cf. moulouyensis or Luciobarbus moulouyensis and that were clustered with other African tetraploid barbels in phylogenetic trees (Machordom et al., 1998; Machordom & Doadrio, 2001a; Tsigenopoulos et al., 2003; Berrebi et al., 2014; Geiger et al., 2014; Yang et al., 2015).
An analysis of body proportions based on Kruskal-Wallis and Mann-Whitney post hoc comparisons was used to detect differences in body shape of the tetraploid populations studied (Luciobarbus sp.) from Moulouya Basin with respect to the species L. maghrebensis, L. rifensis, L. ksibi and L. setivimensis from the nearest basins (Appendix I). No differences in SL were found among Luciobarbus populations. However we found significant differences in all morphometric and meristic variables studied.
The population from Moulouya Basin had the longest barbels of all populations studied, a fact that could be habitat-related. Most rivers from Moulouya Basin have muddy bottoms in contrast to the stony or sand bottoms present in the rivers inhabited by L. rifensis, L. ksibi or L. setivimensis. Our samples of the Zobzite River from Moulouya Basin were an exception, because there is no muddy bottom. However, no significant differences were found in the length of the barbels between samples of Zobzite and the rest of samples of Moulouya Basin.
The samples of L. ksibi and L. setivimensis had the length of the head significantly smaller than L. rifensis, L. maghrebensis and samples of Moulouya Basin as consequence of a shorter snout. In this way, our samples of L. setivimensis and L. ksibi from Oum er Rbia Basin showed a head conspicuously smaller and rounded than the rest. The eye diameter was significantly
longer in Moulouya Basin population with respect to other Luciobarbus species. Due to allometry in fishes, juvenile specimens present an eye bigger in proportion to body size. Nonetheless, all
our samples were mature individuals and no differences in body size among the populations studied were found. The longest
barbels were found in Moulouya Basin population and usually the first barbel reached the half of the eye diameter and the
second barbel reached the preopercular (Fig. 5). The caudal peduncle was lower in L. moulouyensis and L. ksibi than in other Luciobarbus species and shortest in L. setivimensis.
|
 Fig. 5.— Head details of one specimen of Moulouya population of 136 mm SL showing the big develop of the barbels. Fig. 5.— Head details of one specimen of Moulouya population of 136 mm SL showing the big develop of the barbels.
Fig. 5.— Detalles de la cabeza de un ejemplar de 136 mm de SL de la población del Moulouya mostrando el gran desarrollo de las barbillas.
|
|
Number of scales on the lateral line were less numerous in Luciobarbus moulouyensis (Median=43), meanwhile L. ksibi showed more scales in the lateral line (Median=45) and transversal rows (Median RSA=9.48 and Median RSB=6.35) than other Luciobarbus.
The PCA separated the specimens of L. setivimensis of other Luciobarbus species. The remaining populations showed some overlap between them but could be clearly identified in the PCA as differentiated populations. Luciobarbus moulouyensis overlapped L. ksibi and L. maghrebensis (Fig. 6). The eigenvalues of the three first principal components, with the Burnaby-corrected matrix, explained most of the variance (Table 4). The highest eigenvector values (barbels length, size of the fins and body least deep) were in agreement with results of Kruskal–Wallis and Mann–Whitney analyses (Table 4, Appendix 1).
|
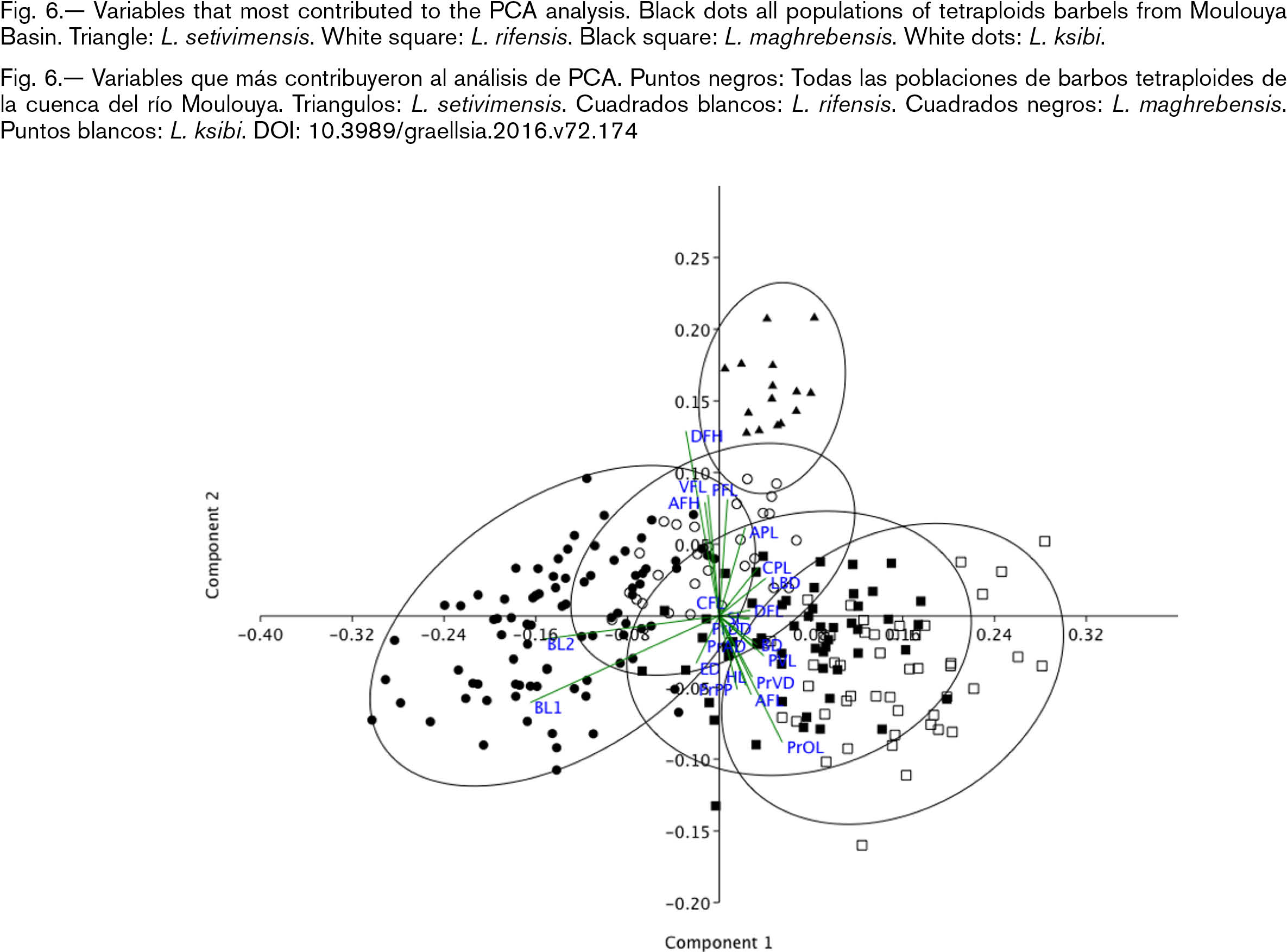 Fig. 6.— Variables that most contributed to the PCA analysis. Black dots all populations of tetraploids barbels from Moulouya Basin. Triangle: L. setivimensis. White square: L. rifensis. Black square: L. maghrebensis. White dots: L. ksibi. Fig. 6.— Variables that most contributed to the PCA analysis. Black dots all populations of tetraploids barbels from Moulouya Basin. Triangle: L. setivimensis. White square: L. rifensis. Black square: L. maghrebensis. White dots: L. ksibi.
Fig. 6.— Variables que más contribuyeron al análisis de PCA. Puntos negros: Todas las poblaciones de barbos tetraploides de la cuenca del río Moulouya. Triangulos: L. setivimensis. Cuadrados blancos: L. rifensis. Cuadrados negros: L. maghrebensis. Puntos blancos: L. ksibi.
|
|
Table 4.— Eigenvalues and eigenvectors for the first three principal components (PC1-PC3) of 22 morphometric variables for all populations studied of Luciobarbus in North Africa. Acronyms are defined in the Material and Methods section. Variables with the highest eigenvalues for each PC are in bold.
Tabla 4.— Eigenvalores y eigenvectores para los tres primeros componentes principales (PC1-PC3) de 22 variables morfométricas para todas las poblaciones estudiadas del género Luciobarbus en el Norte de África. Los acrónimos están definidos en la sección de Material y Métodos. Las variables con los eigenvalores más altos para cada CP están en negrita.
| Variables |
PCI |
PCII |
PCIII |
| Eigenvalue |
0.0162 |
0.0038 |
0.0019 |
| % variance |
52.46 |
12.33 |
6.04 |
| Eigenvectors |
| SL |
0.1031 |
0.0042 |
0.0614 |
| PrDD |
0.1034 |
0.0092 |
0.0524 |
| PrPD |
0.0606 |
0.1989 |
0.0779 |
| PrVD |
0.1118 |
0.1656 |
0.1403 |
| PrAD |
0.1081 |
0.0813 |
0.0949 |
| PVL |
0.1509 |
0.1076 |
0.1799 |
| CPL |
0.1316 |
0.1312 |
0.0237 |
| APL |
0.0874 |
0.2385 |
0.0545 |
| BD |
0.1277 |
0.0779 |
0.1051 |
| BLD |
0.1580 |
0.1021 |
0.0592 |
| HL |
0.0903 |
0.1888 |
0.0359 |
| PrOL |
0.2125 |
0.3416 |
0.0266 |
| ED |
0.0774 |
0.1279 |
0.2710 |
| B1L |
0.6381 |
0.2345 |
0.4552 |
| B2L |
0.5950 |
0.0632 |
0.4363 |
| PFL |
0.0277 |
0.3152 |
0.3151 |
| VFL |
0.0382 |
0.3267 |
0.2215 |
| AFL |
0.1079 |
0.2131 |
0.2332 |
| AFH |
0.0478 |
0.3067 |
0.1997 |
| DFL |
0.1049 |
0.0153 |
0.1932 |
| DFH |
0.1128 |
0.4994 |
0.3868 |
| CFL |
0.0371 |
0.0354 |
0.0746 |
OSTEOLOGICAL FEATURES
The shape of the last unbranched dorsal-fin ray (DFR) has been considered an important diagnostic trait in barbel taxonomy (Doadrio, 1990). A smooth DFR was characteristic of Carasobarbus but as we have previously pointed out, some specimens from Moulouya population had denticulations but in all cases those
denticulations were very small and could be a signal of genetic introgression with Luciobarbus. Reophilic and limnetic barbels can be recognized for the hardness of the DFR. Reophilic barbels have a stronger DFR than
limnetic kind and this can be measured (Doadrio et al., 2016).
Within limnetic barbels L. rifensis and L. maghrebensis had denticulations in practically all the length of the DFR, in contrast to the species L. setivimensis, L. ksibi and the population of Moulouya Basin that had denticulations only in 2/3 of the length of DFR. The number of denticulations, in the DFR from Moulouya Basin was always less than twenty-one (16-20, n=10) but longer than in the other species studied (Appendix 2.1).
The skull of L. setivimensis, L. ksibi and of Moulouya population was wider than that of L. rifensis and L. maghrebensis and this was especially remarked in the interorbital distance (Appendix 2.2). The species L. maghrebensis showed narrower ethmoids than any other species (Appendix 2.2). In a lateral view, the skull was placed higher with respect to its length in Moulouya Basin population with respect to the rest of populations (Appendix 2.3). The opercular was small and was slightly anteriorly projected (Appendix 2.3). The infraorbital bones were wide as in Luciobarbus ksibi and the lachrymal was poorly notched (Appendix 2.4). The dorsal branch of the pharyngeal bone was the longest of all studied populations and scarcely curved (Appendix 2.5). The maxilla and the dentary in Moulouya Basin population showed longer anterior process than the rest of populations (Appendix 2.6). The basioccipital had a triangular and small basal plate (Appendix 2.6). Kinethmoides was as small and wide as in other limnophilic Luciobarbus (Appendix 2.6).
MOLECULAR DATA
Phylogenetic analyses based on the cytb gene supported two main clades in the tree, one corresponding to the populations of Iberian Peninsula, L. setivimensis from Algeria and L. guercifensis from Morocco together; the other one, comprised by the remaining populations of North African (Fig. 7). These phylogenetic relationships are in agreement with previous works (Machordom & Doadrio, 2001b; Doadrio et al., 2016). In the second group comprised by the rest of North African species, L. moulouyensis was basal and the most differentiated from a genetic point of view. The species L. maghrebensis and L. ksibi were sister group to L. rifensis. Uncorrected-p genetic distances of L. moulouyensis with respect to L. maghrebensis, L. rifensis and L. ksibi were greater than 5% (Table 5). With respect to reophilic L. guercifensis inhabiting the same basin, the uncorrected-p genetic distances were x = 8.8%. These distances were of similar range as those between well-recognized species of cyprinid fishes (Doadrio et al., 2002; Doadrio & Carmona, 2003, 2006; Doadrio & Madeira, 2004; Robalo et al., 2005; Doadrio & Elvira, 2007; Doadrio et al., 2007; Domínguez-Domínguez et al., 2007, 2009; Casal-López et al., 2015; Doadrio et al., 2016). These results confirmed the differences found with allozyme analyses (Machordom et al., 1998). Allozyme studies have been useful to distinguish Luciobarbus species especially when diagnostic loci are present (Machordom et al., 1995) and also to describe hybridization between barbel species (Machordom et al., 1990). In a previous work based on 23 polymorphic loci in Luciobarbus species of North Africa, one diagnostic locus (LDH-4*) was found in populations of Luciobarbus from Moulouya Basin with respect to other species or populations from North Africa (Machordom et al., 1998).
|
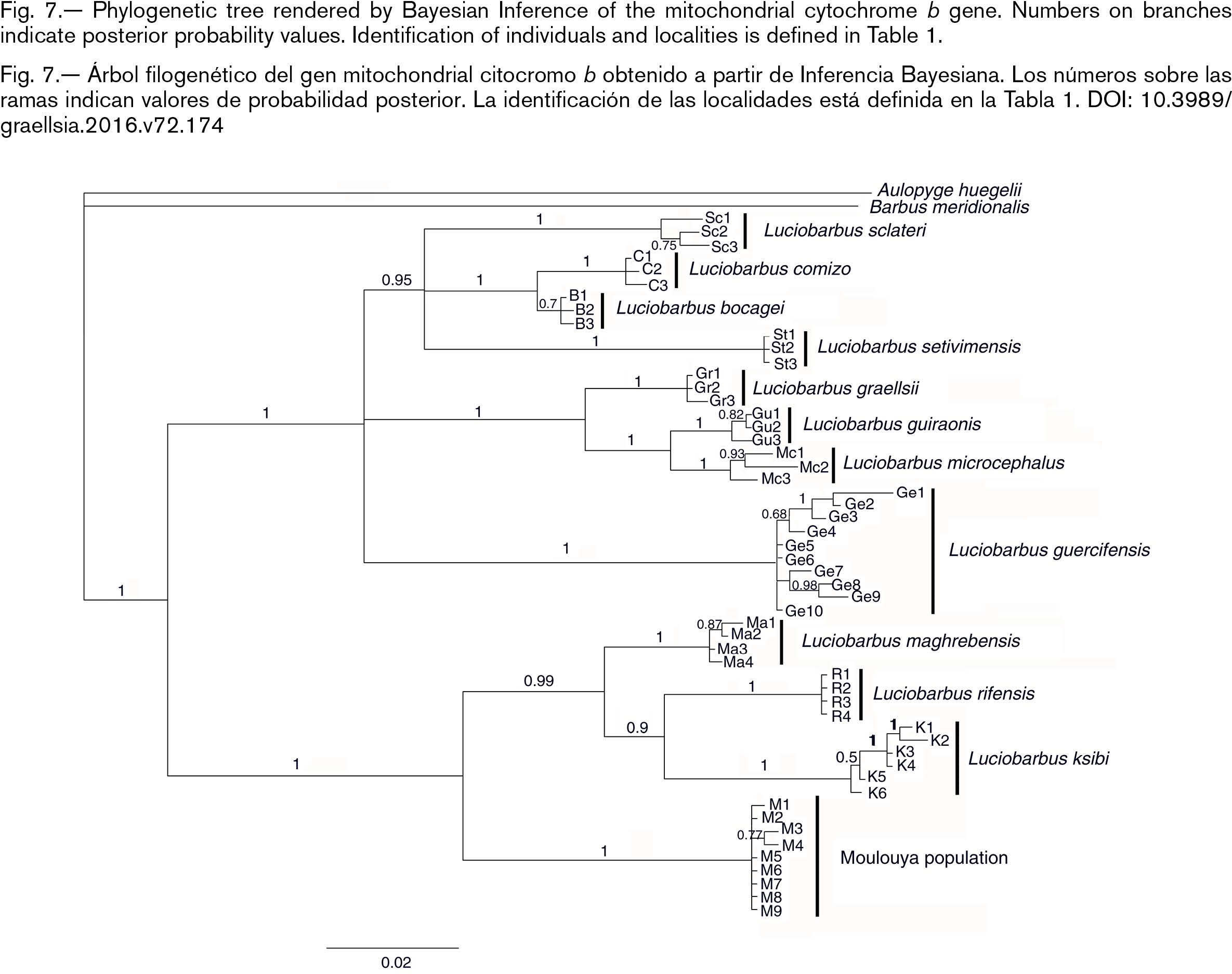 Fig. 7.— Phylogenetic tree rendered by Bayesian Inference of the mitochondrial cytochrome b gene. Numbers on branches indicate posterior probability values. Identification of individuals and localities is defined in Table 1. Fig. 7.— Phylogenetic tree rendered by Bayesian Inference of the mitochondrial cytochrome b gene. Numbers on branches indicate posterior probability values. Identification of individuals and localities is defined in Table 1.
Fig. 7.— Árbol filogenético del gen mitochondrial citocromo b obtenido a partir de Inferencia Bayesiana. Los números sobre las ramas indican valores de probabilidad posterior. La identificación de las localidades está definida en la Tabla 1.
|
|
DESCRIPTION OF LUCIOBARBUS POPULATIONS
The high degree of morphological and genetic differentiation of limnetic populations of the genus Luciobarbus from Moulouya Basin in North Africa justifies the consideration of these populations as a distinct species. No available
name for these populations exists, and therefore one new species is described in this study.
Luciobarbus yahyaouii Doadrio, Casal-López & Perea, sp. nov.
http://urn:lsid:zoobank.org:act:60BBBFE5-4D66-4B34-BF0F-F0B7A7D77CF1
TYPE MATERIAL: Holotype: Fig. 8, Table 6. MNCN_ICTIO 290.958 male, 119.8 mm (SL); Moulouya River, Moulouya Basin, Ghafoula, Mediterranean slope in Morocco (34.13502°N,
3.39653°O) (Fig. 1); 2/V/2015. Collected by (Coll.) Doadrio, I.; Garzón, P.; Yahyaoui, A; Perea, S.
Paratypes: Table 6. MNCN_ICTIO 290.951-290.957, 290.959-290.961: 10 specimens from the Moulouya River, Moulouya Basin, Ghafoula, Mediterranean
slope in Morocco (34.133318, -3.391995), 2/V/2015, Coll. Doadrio, I; Yahyaoui, A.; Garzón, P.; Perea, S. MNCN_ICTIO 290.864-290.878,
290.880-290.885, 290.887-290.991); 26 specimens from Moulouya River, Moulouya Basin, El ksabi, Atlantic slope in Morocco (32.834840,
-4.405431), 19.X.2014, coll. Doadrio, I; Yahyaoui, A.; Garzón, P.; Perea, S. MNCN_ICTIO 290.995-290.997, 290.998-291.006:
12 specimens from the Melloulou River, Moulouya Basin, Guercif, Mediterranean slope in Morocco (34.21526 -3.37568), 2.V.2015,
coll. Doadrio, I; Yahyaoui, A.; Garzón, P.; Perea, S. MNCN_ICTIO 290.910-290.936); 27 specimens from Zobzite River, Moulouya
Basin, Berkine, Atlantic slope in Morocco (33.78631, -3.79980), 19.X.2014, coll. Doadrio, I; Yahyaoui, A.; Garzón, P.; Perea,
S. MNCN_ICTIO 71606-71.611, 71.613-71.614: 8 specimens from Za River, Moulouya Basin, Guefait (type locality of “Barbus” moulouyensis), Atlantic slope in Morocco (33.78631, -3.79980), 21/4/1991, Coll. Doadrio, I; Cubo, J.; Perdices, A.
Holotype and a series of paratypes (83 specimens) have been deposited at the Fish Collection of the Museo Nacional de Ciencias
Naturales, CSIC (Madrid, Spain).
|
 Fig. 8.— Holotype of Luciobarbus yahyaouii from the Moulouya River, Moulouya Basin, Ghafoula, Morocco. MNCN_ICTIO 290.958. Fig. 8.— Holotype of Luciobarbus yahyaouii from the Moulouya River, Moulouya Basin, Ghafoula, Morocco. MNCN_ICTIO 290.958.
Fig. 8.— Holotipo de Luciobarbus yahyaouii del río Moulouya, cuenca del Moulouya, en Ghafoula, Marruecos. MNCN_ICTIO 290.958.
|
|
DIAGNOSIS: Differs from other known species of Luciobarbus by the following combination of characters: 42-45 scales on the lateral line (x =43, Median=43; 8-9 (x =8.1 Median=8) above lateral
line and 5-6 (x =5.3, Median=5) below lateral line. Insertion of the ventral fin is placed in the same edge of the dorsal fin
origin. The last single fin ray is ossified in two/thirds of its length, deeply serrated with teeth in the middle part longer
than the wide of the ray (Fig. 6). Barbels longer than in other studied populations, the first barbel overpasses the preorbital distance (x =1.1 Median=1.1;
range=0.7-1.4) while in other species it does not reach the preorbital distance (L. magrebensis Median= 0.7; L. ksibi Median= 0.8; L. rifensis Median= 0.6; L. setivimensis Median= 0.8). The second barbel usually has the same length that the postorbital distance (x =1 Median=1; range=0.8-1.4) while
in other species it does not reach the postorbital distance (L. magrebensis Median= 0.5; L. ksibi Median= 0.8; L. rifensis Median= 0.6; L. setivimensis Median= 0.7). The ethmoid bone is wider than its length. The dorsal branch of the pharyngeal bone is long and forms an open
angle with respect to the inferior branch. Vertebrae 41-43 (x =41.6, n=10), Gill Rakers (GR) 14-17 (x =15.9 Median=16).
DESCRIPTION: D IV 8, A III 5, P I 15-16, V I 8, C 18; LL 42-45 (x =43, Median=43), RSA 8-9 (x =8.1, Median=8), RSB 5-6 (x =5.3, Median=5). Pharyngeal teeth in adults 4.3.2/4.3.2., GR 14-17 (x =15.9, Median=16), VE 41-43 (x =41.6, n=10). A medium-sized species, rarely reaching 500 mm, females are larger than males. The body is elongated in relation to maximum body depth with BD 22-28% of SL. The head is short with respect to the body with HL 22-24% of SL. Infraorbital bones are narrow. The first barbel reaches the rim of the eye and in several specimens it is extended to half the width of the eye. The second barbel usually extends beyond the posterior rim of the eye, usually reaching the preopercule. The anterior barbel is 22-48% (Median=36%) and the second 36-62% (Median=45% of HL). The lips are thick and exhibit a well-developed medial lobe. The lacrimal bone has a medium-sized manubrium. The snout is prominent, but less marked than in L. maghrebensis and L. rifensis, with preorbital length 6-9% of SL. The iris, as in other Moroccan species of Luciobarbus, is yellowish pigmented at the superior border but less conspicuously than in L. ksibi. The profile of the dorsal fin is straight, with the last single ray ossified in two-thirds of its length and deeply serrated (Appendix 2-1). The caudal peduncle is less deep than in other species studied, with the exception of L. ksibi, and the BLD is 10-12.8% SL. The height of the caudal peduncle is 1.5 to 1.9 times (Median=1.7) the length of the anal peduncle. The pectoral and ventral fins are longer in males, and the anal fin is longer in females. Males exhibit nuptial tubercles in the preorbital space. Ventral fins are inserted on the edge of the dorsal fin insertion. The caudal fin is 19.8-26.7% SL. Morphometric and meristic measurements for the holotype and paratypes of Luciobarbus yahyaouii are represented in Table 6. The coloration of L. maghrebensis is silver or silver-yellowish in fins (Fig. 8). Some specimens exhibit a mid-flank dark stripe. Juveniles present blotches, as in other Luciobarbus species. The skull is wide with a wide ethmoid bone; the pharyngeal bone has a long dorsal process. The lacrimal bone is well developed, and infraorbital bones are wide. The dentary and the maxilla have long anterior processes. The basioccipital plate is small and triangular.
ETYMOLOGY: The species is named after Dr. Ahmed Yahyaoui, who contributed to the knowledge of the fish fauna of Morocco and North Africa.
DISTRIBUTION: This species is endemic to east part of Morocco, inhabiting Moulouya Basin in the Mediterranean slope (Fig. 1).
COMMON NAME: We propose using the English common name “Yahyaoui barbel” for this new species.
HABITAT AND BIOLOGY: The species is ubiquist generally inhabiting rivers with sandy and gravel substrates and in downstream muddy substrates. Luciobarbus yahyaouii is the most common fish species in the Moulouya Basin where are also present other species as the trout (Salmo sp.) in upperstream currents, the North African shad (Alosa algeriensis Regan, 1916) and the sea lamprey (Petromyzon marinus Linnaeus, 1758) in the lower courses of rivers. Another two cyprinid species are known in Moulouya Basin; the scarce and rare species Luciobarbus guercifensis that inhabits riffle areas; and Carasobarbus cf. fritschii that shows habitat requeriments similar to L. yahyaouii but are less abundant than the new described species. From April to May the species migrates upstream for spawning. The species is also present in reservoirs.
CONSERVATION: Luciobarbus yahyaouii is a common species in Moulouya Basin but the populations are declining due to the development of agricultural activity in
the area. As consequence, the Upper Moulouya River has a low regime and poor water quality linked to agriculture. Besides,
the river is regulated downstream where there are both a dam and a reservoir. Therefore our suggestion would be to include
this species in the IUCN category of Near Threatened (NT).
GENETICS: Uncorrected-p distances of mitocondrial gene cytb between L. yahyaouii and the other analysed species are presented in Table 5. Luciobarbus yahyaouii shows one diagnostic locus to (LDH-4*) and 12 diagnostic nucleotide positions to Cytochrome b.
Table 5.— Genetic distances for the complete mitocondrial cytb gene. Uncorrected-p genetic distances between species are below the diagonal. Uncorrected-p genetic distances within species are shown in the diagonal.
Table 5.— Distancias genéticas para el gen mitocondrial citocromo b completo. Las distancias genéticas no corregidas entre especies están debajo de la diagonal. Las distancias genéticas no
corregidas dentro de las especies se muestran en la diagonal.
|
L. maghrebensis |
L. rifensis |
L. ksibi |
L. setivimensis |
L. guercifensis |
Moulouya Basin |
| L. maghrebensis |
0.2 |
|
|
|
|
|
| L. rifensis |
3.6 |
0 |
|
|
|
|
| L. ksibi |
3.9 |
4.0 |
0.4 |
|
|
|
| L. setivimensis |
9.5 |
9.5 |
11.2 |
0 |
|
|
| L. guercifensis |
9.3 |
9.1 |
10.5 |
7.6 |
0.6 |
|
| Moulouya Basin |
5.6 |
5.7 |
6.8 |
9.6 |
8.8 |
0.1 |
Table 6.— Morphometric and meristic measurements of the holotype and paratypes of Luciobarbus yahyaouii.
Tabla 6.— Medidas morfométricas y merísticas del holotipo y paratipos de Luciobarbus yahyaouii.
| Morphometric variables |
Holotype MNCN_ICTIO 290.958 |
Paratypes n= 83 |
| Measurements (mm) |
Mean |
Range |
Standard deviation |
| SL |
119.8 |
110.7 |
182.7–64.3 |
27.8 |
| HL |
30.2 |
27.3 |
44.3–16.3 |
6.6 |
| PrOL |
9.6 |
9.1 |
16.1–4.6 |
2.6 |
| ED |
6.5 |
6 |
8.4–4.2 |
1.1 |
| PsOL |
13.7 |
12.4 |
19.7–7.2 |
3 |
| BL1 |
12 |
9.9 |
17.8–3.7 |
3.4 |
| BL2 |
15.2 |
12.7 |
20.7–6.1 |
3.5 |
| PrDD |
63.2 |
58.6 |
95.6–34.7 |
14.1 |
| PrPD |
33.1 |
29.1 |
47.5–16.4 |
7.5 |
| PrVD |
65.8 |
59.8 |
104.2–33.8 |
15.7 |
| PrAD |
90.5 |
83.9 |
143.6–47.3 |
21.9 |
| CPL |
43.5 |
40 |
65.6–22.8 |
10.3 |
| APL |
22.4 |
20.9 |
34.1–12.9 |
5 |
| PVL |
34.7 |
31 |
54.1–16.5 |
8.3 |
| BD |
28.3 |
27.4 |
45.6–15.6 |
6.8 |
| BLD |
12.7 |
12.3 |
19.5–7.5 |
2.8 |
| DFL |
15.8 |
15.3 |
23.8–9.1 |
3.7 |
| DFH |
25.5 |
21.3 |
34–12.3 |
5.1 |
| PFL |
23.5 |
20.9 |
33.3–9.8 |
5.5 |
| VFL |
20.3 |
18.9 |
30.1–11.7 |
4.5 |
| AFL |
8.8 |
9 |
15.7–4.8 |
2.6 |
| AFH |
23.6 |
20.7 |
33.1–12.5 |
5.3 |
| CFL |
25.6 |
25.5 |
40.6–15.2 |
5.9 |
| LL |
43 |
43 |
45–42 |
0.8 |
| RSA |
8 |
8.1 |
9–8 |
0.3 |
| RSB |
5 |
5.3 |
6–5 |
0.5 |
AcknowledgmentsTOP
We thank to J. Cubo, M. Merino, J. L. González, P. Garzón, I. Doadrio Jr., A. Doadrio, A. Perdices, Y. Bernat, and S. El Gharbi
for the their help in field during these last years. We would also like to thank L. Alcaraz, involved in lab work, the curator
of the ichthyological collection of National Museum of the Natural Sciences (MNCN-CSIC), G. Solís and also C. Parejo for her
technical assistance at the lab of non-destructive techniques in the computerized Tomography scan (CTscan) at the MNCN-CSIC.
This project was funded by MESRSFC and the CNRST from Morocco to the Project N°PPR/2015/2 “Impact des changements climatiques
sur la diversité génétique des poissons des eaux douces du Maroc”. Permission for fish collection was provided by the High
Commissioner for Water, Forest and the Fight Against Dessertification (HCEFLCD) of Morocco.
ReferencesTOP
| ○ |
Akaike, H., 1973. Information theory and an extension of the Maximum Likelihood principle. In: B. N. Petrov & F. Csaki (eds.).
Proceedings of the second International Symposium on Information Theory. Budapest: 267-281.
|
| ○ |
Almaça, C., 1966. Sur la systématique des Barbeaux marocaines (Pisces, Cyprinidae, Barbus). Arquivos do Museo Bocage, 7: 111-114.
|
| ○ |
Almaça, C., 1968. Revision critique de quelques types de Cyprinidés d’Europe et d’Afrique du Nord des collections du Muséum
National d’Histoire Naturelle. Bulletin du Muséum National d’Historie Naturelle, 40: 1116-1144.
|
| ○ |
Almaça, C., 1970. Sur les barbeaux (genre et sous-genre Barbus) de l’Afrique du Nord. Bulletin du Muséum National d’Historie Naturelle, 42: 141-158.
|
| ○ |
Berrebi, P., Chenuil, A., Kotlík, P., Machordom, A. & Tsigenopoulos, C. S., 2014. Disentangling the evolutionary history of
the genus Barbus sensu lato, a twenty years adventure. In: M. J. Alves, A. Cartaxana, A. M. Correia & L. F. Lopes (coords.). Professor Carlos Almaça (1934–2010)–Estado da arte em áreas científicas do seu interesse. Museu Nacional de História Natural e da Ciência. Lisboa: 29-55.
|
| ○ |
Beshera, K. A., Harris, P. M. & Mayden, R. L., 2016. Novel evolutionary lineages in Labeobarbus (Cypriniformes; Cyprinidae) based on phylogenetic analyses of mtDNA sequences. Zootaxa, 4093(3): 363-381. http://dx.doi.org/10.11646/zootaxa.4093.3.4 |
| ○ |
Borkenhagen, K. & Krupp, F., 2013. Taxonomic revision of the genus Carasobarbus Karaman, 1971 (Actinopterygii, Cyprinidae). ZooKeys, 339: 1-53. http://dx.doi.org/10.3897/zookeys.339.4903 |
| ○ |
Burnaby, T. P., 1966. Growth-invariant discriminant functions and generalized distances. Biometrics, 22: 96-110. http://dx.doi.org/10.2307/2528217 |
| ○ |
Casal-López, M., Perea, S., Yahyaoui, A. & Doadrio, I., 2015. Taxonomic review of the genus Luciobarbus Heckel, 1843 (Actinopterygii, Cyprinidae) from Northwestern Morocco with the description of three new species. Graellsia, 71(2): e027. http://dx.doi.org/10.3989/graellsia.2015.v71.135 |
| ○ |
Doadrio, I., 1990. Phylogenetics relationships and classification of western paleartic species of the genus Barbus (Osteichthyes, Cyprinidae). Aquatic Living Resources, 3: 265-282.
|
| ○ |
Doadrio, I., 1994. Freshwater fish fauna of North Africa and its biogeography. Annals of the Royal Central African Museum (Zoology), 275: 21-34.
|
| ○ |
Doadrio, I. & Carmona, J. A., 2003. A new species of the genus Chondrostoma Agassiz, 1832 (Actinopterygii, Cyprinidae) from the Iberian Peninsula. Graellsia, 59(1): 29-36. http://dx.doi.org/10.3989/graellsia.2003.v59.i1.221 |
| ○ |
Doadrio, I. & Carmona, J. A., 2006. Phylogenetic overview of the genus Squalius (Actinopterygii, Cyprinidae) in the Iberian Peninsula, with description of two new species. Cybium, 30(3): 199-214.
|
| ○ |
Doadrio, I., Carmona, J. A. & Machordom, A., 2002. Haplotypes diversity and phylogenetic relationships among the Iberian barbels
(Barbus, Cyprinidae) reveal two evolutionary lineages. Journal of Heredity, 93(2): 140-147. http://dx.doi.org/10.1093/jhered/93.2.140 |
| ○ |
Doadrio, I., Casal-López, M., Perea, S., & Yahyaoui, A., 2016. Taxonomy of rheophilic Luciobarbus Heckel, 1842 (Actinopterygii, Cyprinidae) from Morocco with the description of two new species. Graellsia, 72(1): e039. http://dx.doi.org/10.3989/graellsia.2016.v72.153 |
| ○ |
Doadrio, I. & Elvira, B., 2007. A new species of the genus Achondrostoma Robalo, Almada, Levy & Doadrio, 2007 (Actinopterygii, Cyprinidae) from western Spain. Graellsia, 63(2): 295-304. http://dx.doi.org/10.3989/graellsia.2007.v63.i2.96 |
| ○ |
Doadrio, I. & Madeira, M. J., 2004. A new species of the genus Gobio Cuvier 1816 (Actynopterigii, Cyprinidae) from the Iberian Peninsula and south of France. Graellsia, 60(1): 107-116. http://dx.doi.org/10.3989/graellsia.2004.v60.il.197 |
| ○ |
Doadrio, I., Perea, S. & Alonso, F., 2007. A new species of the genus Squalius Bonaparte, 1837 (Actinipterygii, Cyprinidae) from the Tagus River Basin (Central Spain). Graellsia, 63(1): 89-100. http://dx.doi.org/10.3989/graellsia.2007.v63.i1.83 |
| ○ |
Domínguez-Domínguez, O., Pérez-Rodríguez, R., Escalera-Vázquez, L. H. & Doadrio, I., 2009. Two new species of the genus Notropis Rafinesque, 1817 (Actinopterygii, Cyprinidae) from the Lerma River Basin in Central Mexico. Hidrobiológica, 19(2): 159-172.
|
| ○ |
Domínguez-Domínguez, O., Pompa-Domínguez, A. & Doadrio, I., 2007. A new species of the genus Yuriria Jordan & Evermann, 1896 (Actinopterygii, Cyprinidae) from the Ameca Basin of the Central Mexican Plateau. Graellsia, 63(2): 259-271. http://dx.doi.org/10.3989/graellsia.2007.v63.i2.93 |
| ○ |
Estève, R., 1947. Étude biométrique des barbeaux marocains. Bulletin du Muséum National d’Histoire Naturelle, 3: 265-270.
|
| ○ |
Geiger, M. F., Herder, F., Monaghan, F. T., Almada, V., Barbieri, R., Bariche, M., Berrebi, P., Bohlen, J., Casal-López, M.,
Delmastro, G. B., Denys, G. P. J., Dettai, A., Doadrio, I., Kalogianni, E., Kärst, H., Kottelat, A., Kovačić, M., Laporte,
M., Lorenzoni, M., Marčić, Z., Özuluğ, M., Perdices, A., Perea, S., Persat, H., Porcelotti, S., Puzzi, C., Robalo, J., Šanda,
R., Schneider, M., Šlechtová, V., Stumboudi, M., Walter, S. & Freyhof, J., 2014. Spatial heterogeneity in the Mediterranean
biodiversity hotspot affects barcoding accuracy of its freshwater fishes. Molecular Ecology Resources, 14: 1210-1221. http://dx.doi.org/10.1111/1755-0998.12257 |
| ○ |
Hammer, Ø., Harper, D. A. T. & Ryan, P. D., 2001. PAST: Paleontological statistics software package for education and data
analysis, 2011. Paleontologia Electronica, 4: 9 pp.
|
| ○ |
Levin, B. A., Freyhof, J., Lajbner, Z., Perea, S., Abdoli, A., Gaffaroğlu, M., Özulug, M., Rubenyan, H. R., Salnikov, V. B.
& Doadrio, I., 2012. Phylogenetic relationships of the algae scraping cyprinid genus Capoeta (Teleostei: Cyprinidae). Molecular Phylogenetics and Evolution, 62(1): 542-549. http://dx.doi.org/10.1016/j.ympev.2011.09.004 |
| ○ |
Machordom, A., Berrebi, P. & Doadrio, I., 1990. Spanish barbel hybridization detected using enzymatic markers: Barbus meridionalis Risso × Barbus haasi Mertens (Osteichthyes, Cyprinidae). Aquatic Living Resources, 3(4): 295-303.
|
| ○ |
Machordom, A., Bouhadad, I. & Doadrio, I., 1998. Allozyme variation and evolutionary history of North African populations
of the genus Barbus (Osteichthyes, Cyprinidae). Diversity and Distributions, 4: 217-234
|
| ○ |
Machordom, A. & Doadrio, I., 2001a. Evolutionary history and speciation modes in the cyprinid genus Barbus. Proceedings of the Royal Society of London. Series B: Biological Sciences, 268(1473): 1297-1306. http://dx.doi.org/10.1098/rspb.2001.1654 |
| ○ |
Machordom, A. & Doadrio, I., 2001b. Evidence of a Cenozoic-Betic-Kabilian connection based on freshwater fish phylogeography
(Luciobarbus, Cyprinidae). Molecular Phylogenetics and Evolution, 18(2): 252-263. http://dx.doi.org/10.1006/mpev.2000.0876 |
| ○ |
Machordom, A., Doadrio, I., & Berrebi, P., 1995. Phylogeny and evolution of the genus Barbus in the Iberian Peninsula as revealed by allozyme electrophoresis. Journal of Fish Biology, 47(2): 211-236. http://dx.doi.org/10.1111/j.1095-8649.1995.tb01890.x |
| ○ |
Pellegrin, J., 1921. Les poissons des eaux douces de l’Afrique du Nord Française: Maroc, Algérie, Tunisie, Sahara. Memoires de la Société des Sciences Naturelles du Maroc,1: 1–216.
|
| ○ |
Pellegrin, J., 1924. Batraciens et poissons du Maroc oriental recueillis par M. Ch. Alluaud. Description d’un Barbeau nouveau.
Bulletin de la Société Zoologique de France, v. 49: 457-461.
|
| ○ |
Pellegrin, J., 1930. Variété nouvelle de Barbeau du Maroc. Bulletin du Muséum National d’Histoire Naturelle, Série 2, 2 (6): 623-624.
|
| ○ |
Pellegrin, J., 1939. Les barbeaux de l’Afrique du Nord Française: description d’une espèce nouvelle. Bulletin de la Société des Sciences Naturelles du Maroc, 19(1): 1-10.
|
| ○ |
Posada, D., 2008. jModelTest: Phylogenetic Model Averaging. Molecular Biology and Evolution, 25(7): 1253-1256. http://dx.doi.org/10.1093/molbev/msn083 |
| ○ |
Robalo, J. I., Almada, V. C., Sousa Santos, C., Moreira, M. I. & Doadrio, I., 2005. New species of the genus Chondrostoma Agassiz, 1832 (Actinopterygii, Cyprinidae) from western Portugal. Graellsia, 61(1): 15-28. http://dx.doi.org/10.3989/graellsia.2005.v61.i1.3 |
| ○ |
Rohlf, F. J. & Bookstein, F. L., 1987. A comment on shearing as a method for “size correction”. Systematic Zoology, 36(4): 356-367.
|
| ○ |
Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D. L., Darling, A., Höhna, S., Larget, B., Liu, L., Suchard, M. A. &
Huelsenbeck, J. P., 2012. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choise Across Large Model Space.
Systematic Biology, 61(3): 539-542. http://dx.doi.org/10.1093/sysbio/sys029 |
| ○ |
Tamura, K., Stecher, G., Peterson, D., Filipski, A. & Kumar, S., 2013. MEGA6: Molecular Evolutionary Genetics Analysis (MEGA)
Software version 6.0. Molecular Biology and Evolution, 24(8): 1596-1599.
|
| ○ |
Tsigenopoulos, C. S., Durand, J. D., Ünlu, E. & Berrebi, P., 2003. Rapid radiation of the Mediterranean Barbus species (Cyprinidae) after the Messinian Salinity Crisis of the Mediterranean Sea, inferred from mitochondrial phylogenetic
analysis. Biological Journal of the Linnean Society, 80(2): 207-222. http://dx.doi.org/10.1046/j.1095-8312.2003.00237.x |
| ○ |
Yang, L., Sado, T., Vincent Hirt, M., Pasco-Viel, E., Arunachalam, M., Li, J., Wang, X., Freyhof, J., Saitoh, K., Simons,
A. M., Miya, M., He, S. & Mayden, R. L., 2015. Phylogeny and polyploidy: resolving the classification of cyprinine fishes
(Teleostei: Cypriniformes). Molecular Phylogenetics and Evolution, 85: 97-116.
|
| ○ |
Zardoya, R. & Doadrio, I., 1999. Molecular evidence on the evolutionary and biogeographical patterns of European cyprinids.
Journal of Molecular Evolution, 49(2): 227-237.
|
APPENDIXTOP
Appendix 1.— Kruskal-Wallis test and Non-parametric Mann-Whitney’s pairwise comparisons for all populations. Values of Kruskal-Wallis test (H) below variables. Values of Mann–Whitney test are below the diagonal. Median in the diagonal of each variable. Significant differences p<0.05 (*); p<0.01 (**). Acronyms are defined in the Material and Methods.
Apéndice 1.— Test de Kruskal-Wallis y análisis no paramétrico de Mann-Whitney para todas las poblaciones. Valores para el test de Kruskal-Wallis (H) debajo de las variables. Valores de Mann-Whitney por debajo de la diagonal. Valor de la Mediana en la diagonal de cada variable. Diferencias significativas p<0,05 (*); p<0,01 (**). Las abreviaturas están descritas en el epígrafe de Material y Métodos.
| Variables |
Populations |
Moulouya Basin (n=12) |
L. rifensis (n=12) |
L. maghrebensis (n= 27) |
L. setivimensis (n=8) |
L. ksibi (n=15) |
SL
(H=4.36) |
Moulouya Basin |
110.8 |
|
|
|
|
|
L. rifensis
|
0.254 |
130,67 |
|
|
|
|
L. maghrebensis |
0.456 |
0.46 |
116.27 |
|
|
|
L. setivimensis |
0.361 |
0.73 |
0.869 |
113.15 |
|
|
L. ksibi |
0.079 |
0.494 |
0.361 |
0.322 |
117.9 |
SL/HL
(H=58.48**) |
Moulouya Basin |
4.05 |
|
|
|
|
|
L. rifensis
|
0.850 |
4.04 |
|
|
|
|
L. maghrebensis |
0.662 |
0.872 |
4.02 |
|
|
|
L. setivimensis |
<0.001** |
<0.001** |
<0.001** |
4.28 |
|
|
L. ksibi |
<0.001** |
<0.001** |
<0.001** |
0.843 |
4.28 |
SL/PrO
(H=97.41**) |
Moulouya Basin |
12.24 |
|
|
|
|
|
L. rifensis
|
<0.001** |
11.01 |
|
|
|
|
L. maghrebensis |
<0.001** |
0.022* |
11.32 |
|
|
|
L. setivimensis |
0.003** |
<0.001** |
<0.001** |
12.75 |
|
|
L. ksibi |
0.0223* |
<0.001** |
<0.001** |
0.603 |
12.65 |
SL/OD
(H=34.62**) |
Moulouya Basin |
18.22 |
|
|
|
|
|
L. rifensis
|
<0.001** |
20.07 |
|
|
|
|
L. maghrebensis |
<0.001** |
0.83 |
20.5 |
|
|
|
L. setivimensis |
0.001** |
0.882 |
0.989 |
20.02 |
|
|
L. ksibi |
0.028* |
0.033* |
0.068 |
0.063 |
19.18 |
SL/LB1
(H=130.5**) |
Moulouya Basin |
11.77 |
|
|
|
|
|
L. rifensis
|
<0.001** |
18.57 |
|
|
|
|
L. maghrebensis |
<0.001** |
<0.001** |
15.92 |
|
|
|
L. setivimensis |
<0.001** |
0.079 |
0.115 |
16.45 |
|
|
L. ksibi |
<0.001** |
<0.001** |
0.011* |
0.001** |
14.75 |
SL/L2B
(H=163.4**) |
Moulouya Basin |
8.84 |
|
|
|
|
|
L. rifensis
|
<0.001** |
14.16 |
|
|
|
|
L. maghrebensis |
<0.001** |
<0.001** |
12.41 |
|
|
|
L. setivimensis |
<0.001** |
0.002** |
0.841 |
12.19 |
|
|
L. ksibi |
<0.001** |
<0.001** |
0.024* |
0.102 |
11.59 |
SL/PrD
(H=84.94**) |
Moulouya Basin |
1.89 |
|
|
|
|
|
L. rifensis
|
0.331 |
1.87 |
|
|
|
|
L. maghrebensis |
<0.001** |
<0.001** |
1.99 |
|
|
|
L. setivimensis |
0.038* |
0.081 |
<0.001** |
1.85 |
|
|
L. ksibi |
<0.001** |
<0.001** |
<0.001** |
<0.001** |
1.98 |
SL/PrP
(H=41.67**) |
Moulouya Basin |
3.81 |
|
|
|
|
|
L. rifensis
|
0.239 |
3.83 |
|
|
|
|
L. maghrebensis |
0.031* |
0.375 |
3.88 |
|
|
|
L. setivimensis |
<0.001** |
<0.001** |
<0.001** |
4.11 |
|
|
L. ksibi |
<0.001** |
0.009** |
0.155 |
<0.001** |
3.93 |
SL/PrV
(H=44.95**) |
Moulouya Basin |
1.86 |
|
|
|
|
|
L. rifensis
|
0.002** |
1.82 |
|
|
|
|
L. maghrebensis |
0.015* |
0.368 |
1.83 |
|
|
|
L. setivimensis |
<0.001** |
<0.001** |
<0.001** |
1.95 |
|
|
L. ksibi |
0.213 |
0.045* |
0.361 |
<0.001** |
1.84 |
SL/PrA
(H=51.52**) |
Moulouya Basin |
1.32 |
|
|
|
|
|
L. rifensis
|
<0.006** |
1.31 |
|
|
|
|
L. maghrebensis |
<0.006** |
0.93 |
1.31 |
|
|
|
L. setivimensis |
<0.001** |
<0.001** |
<0.001** |
1.36 |
|
|
L. ksibi |
<0.001** |
0.305 |
0.204 |
<0.001** |
1.3 |
SL/BD
(H=20.97**) |
Moulouya Basin |
4.04 |
|
|
|
|
|
L. rifensis
|
0.0012** |
3.92 |
|
|
|
|
L. maghrebensis |
0.381 |
0.03* |
4.01 |
|
|
|
L. setivimensis |
0.918 |
0.028* |
0.596 |
4.02 |
|
|
L. ksibi |
0.057 |
<0.001** |
0.011* |
0.083 |
4.12 |
SL/BLD
(H=43.37**) |
Moulouya Basin |
9 |
|
|
|
|
|
L. rifensis
|
0.053* |
8.84 |
|
|
|
|
L. maghrebensis |
0.004** |
0.127 |
8.72 |
|
|
|
L. setivimensis |
<0.001** |
<0.001** |
0.002** |
8.27 |
|
|
L. ksibi |
0.288 |
0.002** |
<0.001** |
<0.001** |
9.11 |
SL/CPL
(H=77.5**) |
Moulouya Basin |
2.77 |
|
|
|
|
|
L. rifensis
|
0.117 |
2.75 |
|
|
|
|
L. maghrebensis |
<0.001** |
0.017* |
2.7 |
|
|
|
L. setivimensis |
<0.001** |
<0.001** |
<0.001** |
2.57 |
|
|
L. ksibi |
<0.001** |
<0.001** |
0.002** |
0.013* |
2.63 |
SL/APL
(H=55.87**) |
Moulouya Basin |
5.28 |
|
|
|
|
|
L. rifensis
|
0.022* |
5.41 |
|
|
|
|
L. maghrebensis |
<0.001** |
0.02* |
5.54 |
|
|
|
L. setivimensis |
<0.001** |
<0.001** |
<0.001** |
4.72 |
|
|
L. ksibi |
0.045* |
0.839 |
0.135 |
<0.001** |
5.43 |
SL/PVL
(H=52.61**) |
Moulouya Basin |
3.58 |
|
|
|
|
|
L. rifensis
|
<0.001** |
3.47 |
|
|
|
|
L. maghrebensis |
<0.001** |
0.337 |
3.45 |
|
|
|
L. setivimensis |
0.88 |
0.017* |
0.003** |
3.57 |
|
|
L. ksibi |
<0.001** |
<0.001** |
<0.001** |
0.001** |
3.79 |
SL/DFL
(11.41*) |
Moulouya Basin |
7.25 |
|
|
|
|
|
L. rifensis
|
0.211 |
7.36 |
|
|
|
|
L. maghrebensis |
0.256 |
0.06 |
7.01 |
|
|
|
L. setivimensis |
0.388 |
0.172 |
0.864 |
7.2 |
|
|
L. ksibi |
0.008** |
0.231 |
0.02* |
0.006** |
7.47 |
SL/DFH
(139.2**) |
Moulouya Basin l |
5.18 |
|
|
|
|
|
L. rifensis
|
<0.001** |
6.1 |
|
|
|
|
L. maghrebensis |
<0.001** |
0.778 |
6.11 |
|
|
|
L. setivimensis |
<0.001** |
<0.001** |
<0.001** |
4.7 |
|
|
L. ksibi |
0.452 |
<0.001** |
<0.001** |
<0.001** |
5.22 |
SL/PFL
(H=67**) |
Melloulou |
5.33 |
|
|
|
|
|
L. rifensis
|
<0.001** |
5.77 |
|
|
|
|
L. maghrebensis |
0.422 |
<0.001** |
5.36 |
|
|
|
L. setivimensis |
<0.001** |
<0.001** |
<0.001** |
4.6 |
|
|
L. ksibi |
0.961 |
<0.001** |
0.43 |
<0.001** |
5.29 |
SL/VFL
(H=104.7**) |
Moulouya Basin |
5.85 |
|
|
|
|
|
L. rifensis
|
<0.001** |
6.77 |
|
|
|
|
L. maghrebensis |
0.003** |
<0.001** |
6.08 |
|
|
|
L. setivimensis |
<0.001** |
<0.001** |
<0.001** |
5.42 |
|
|
L. ksibi |
0.009** |
<0.001** |
0.785 |
<0.001** |
6.05 |
SL/AFL
(H=29.11**) |
Moulouya Basin |
12.44 |
|
|
|
|
|
L. rifensis
|
0.557 |
12.59 |
|
|
|
|
L. maghrebensis |
0.539 |
0.317 |
12.34 |
|
|
|
L. setivimensis |
0.001** |
0.011* |
0.005** |
13.28 |
|
|
L. ksibi |
<0.001** |
<0.001** |
<0.001** |
0.674 |
13.45 |
SL/AFH
(H=113.5**) |
Moulouya Basin |
5.37 |
|
|
|
|
|
L. rifensis
|
<0.001** |
6.18 |
|
|
|
|
L. maghrebensis |
<0.001** |
<0.001** |
5.58 |
|
|
|
L. setivimensis |
<0.001** |
<0.001** |
<0.001** |
4.87 |
|
|
L. ksibi |
0.063 |
<0.001** |
0.201 |
<0.001** |
5.49 |
SL/CFL
(H=47.84**) |
Melloulou |
4.34 |
|
|
|
|
|
L. rifensis
|
<0.001** |
4.66 |
|
|
|
|
L. maghrebensis |
0.016** |
<0.001** |
4.23 |
|
|
|
L. setivimensis |
0.064 |
<0.001** |
0.886 |
4.21 |
|
|
L. ksibi |
<0.001** |
0.227 |
<0.001** |
<0.001** |
4.53 |
CPL/BLD
(H=27.1**) |
Moulouya Basin |
3.25 |
|
|
|
|
|
L. rifensis
|
0.497 |
3.22 |
|
|
|
|
L. maghrebensis |
0.534 |
1 |
3.24 |
|
|
|
L. setivimensis |
0.404 |
0.882 |
1 |
3.21 |
|
|
L. ksibi |
<0.001** |
<0.001** |
<0.001** |
<0.001** |
3.47 |
APL/BLD
(H=65.05**) |
Moulouya Basin |
1.71 |
|
|
|
|
|
L. rifensis
|
<0.001** |
1.64 |
|
|
|
|
L. maghrebensis |
<0.001** |
0.001** |
1.58 |
|
|
|
L. setivimensis |
0.012* |
<0.001** |
<0.001** |
1.75 |
|
|
L. ksibi |
0.162 |
0.097 |
<0.001** |
0.004** |
1.68 |
LB1/OD
(H=73.81**) |
Moulouya Basin |
1.6 |
|
|
|
|
|
L. rifensis
|
<0.001** |
1.12 |
|
|
|
|
L. maghrebensis |
<0.001** |
<0.001** |
1.3 |
|
|
|
L. setivimensis |
<0.001** |
0.087 |
0.148 |
1.23 |
|
|
L. ksibi |
<0.001** |
<0.001** |
0.977 |
0.092 |
1.32 |
LB2/OD
(H=94.86**) |
Moulouya Basin |
2.08 |
|
|
|
|
|
L. rifensis
|
<0.001** |
1.46 |
|
|
|
|
L. maghrebensis |
<0.001** |
<0.001** |
1.68 |
|
|
|
L. setivimensis |
<0.001** |
0.022* |
0.668 |
1.65 |
|
|
L. ksibi |
<0.001** |
0.003** |
0.91 |
0.96 |
1.67 |
PrVD/PrDD
(H=72.93**) |
Moulouya Basin |
1.02 |
|
|
|
|
|
L. rifensis
|
0.262 |
1.03 |
|
|
|
|
L. maghrebensis |
<0.001** |
0.007** |
1.06 |
|
|
|
L. setivimensis |
<0.001** |
<0.001** |
<0.001** |
0.95 |
|
|
L. ksibi |
<0.001** |
<0.001** |
0.218 |
<0.001** |
1.08 |
HL/PrO
(H=75.35**) |
Moulouya Basin |
3.02 |
|
|
|
|
|
L. rifensis
|
<0.001** |
2.72 |
|
|
|
|
L. maghrebensis |
<0.001** |
0.021* |
2.82 |
|
|
|
L. setivimensis |
0.51 |
<0.001** |
0.001** |
2.98 |
|
|
L. ksibi |
0.163 |
<0.001** |
0.02* |
0.638 |
2.95 |
PrDL/CPL
(H=87.22**) |
Moulouya Basin |
1.47 |
|
|
|
|
|
L. rifensis
|
0.541 |
1.46 |
|
|
|
|
L. maghrebensis |
<0.001** |
<0.001** |
1.4 |
|
|
|
L. setivimensis |
<0.001** |
<0.001** |
0.548 |
1.39 |
|
|
L. ksibi |
<0.001** |
<0.001** |
<0.001** |
0.003** |
1.33 |
PrAL/APL
(H=56.12**) |
Moulouya Basin |
3.99 |
|
|
|
|
|
L. rifensis
|
0.012* |
4.13 |
|
|
|
|
L. maghrebensis |
<0.001** |
0.075 |
4.24 |
|
|
|
L. setivimensis |
<0.001** |
<0.001** |
<0.001** |
3.46 |
|
|
L. ksibi |
<0.013** |
0.789 |
0.3 |
<0.001** |
4.17 |
LL
(H=78.72**) |
Moulouya Basin |
43 |
|
|
|
|
|
L. rifensis
|
<0.001** |
44 |
|
|
|
|
L. maghrebensis |
<0.001** |
0.043 |
44 |
|
|
|
L. setivimensis |
<0.001** |
0.108 |
0.009** |
44 |
|
|
L. ksibi |
<0.001** |
<0.001** |
<0.001** |
0.16 |
45 |
RSA
(H=179.6**) |
Moulouya Basin |
8 |
|
|
|
|
|
L. rifensis
|
<0.001** |
8.5 |
|
|
|
|
L. maghrebensis |
<0.001** |
<0.001** |
8 |
|
|
|
L. setivimensis |
0.64 |
<0.001** |
<0.001** |
8 |
|
|
L. ksibi |
<0.001** |
<0.001** |
<0.001** |
<0.001** |
9 |
RSB
(H=114.2**) |
Moulouya Basin |
5 |
|
|
|
|
|
L. rifensis
|
<0.001** |
4.5 |
|
|
|
|
L. maghrebensis |
<0.001** |
<0.001** |
5.5 |
|
|
|
L. setivimensis |
<0.001** |
<0.001** |
<0.001** |
6 |
|
|
L. ksibi |
<0.001** |
<0.001** |
<0.001** |
0.028* |
6 |
|
 Appendix 2.1.— Last unbranched dorsal-fin ray in adult specimens (SL>120 mm) of the studied populations. A: Luciobarbus rifensis (Laou River). B: Luciobarbus maghrebensis (Ifrane River). C: Luciobarbus setivimensis (Soummam River). D: Luciobarbus ksibi (Kasab River). E: Moulouya population (Moulouya River, El Ksabi). Appendix 2.1.— Last unbranched dorsal-fin ray in adult specimens (SL>120 mm) of the studied populations. A: Luciobarbus rifensis (Laou River). B: Luciobarbus maghrebensis (Ifrane River). C: Luciobarbus setivimensis (Soummam River). D: Luciobarbus ksibi (Kasab River). E: Moulouya population (Moulouya River, El Ksabi).
Apéndice 2.1.— Último radio no ramificado de la aleta dorsal en ejemplares adultos (SL>120 mm) de las poblaciones estudiadas. A: Luciobarbus rifensis (Laou River). B: Luciobarbus maghrebensis (Ifrane River). C: Luciobarbus setivimensis (Soummam River). D: Luciobarbus ksibi (Kasab River). E: Población del Moulouya (Moulouya River, El Ksabi).
|
|
|
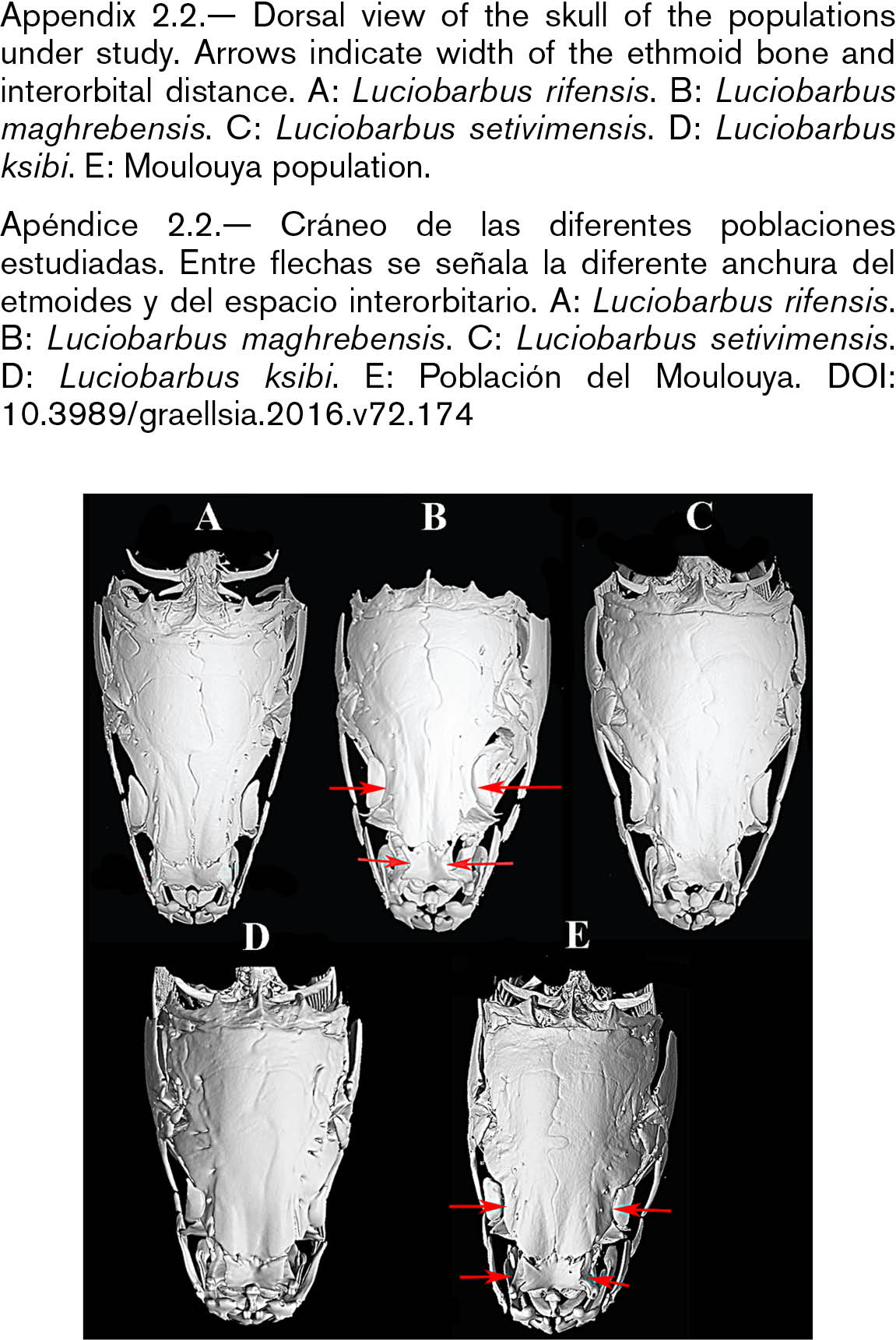 Appendix 2.2.— Dorsal view of the skull of the populations under study. Arrows indicate width of the ethmoid bone and interorbital
distance. A: Luciobarbus rifensis. B: Luciobarbus maghrebensis. C: Luciobarbus setivimensis. D: Luciobarbus ksibi. E: Moulouya population. Appendix 2.2.— Dorsal view of the skull of the populations under study. Arrows indicate width of the ethmoid bone and interorbital
distance. A: Luciobarbus rifensis. B: Luciobarbus maghrebensis. C: Luciobarbus setivimensis. D: Luciobarbus ksibi. E: Moulouya population.
Apéndice 2.2.— Cráneo de las diferentes poblaciones estudiadas. Entre flechas se señala la diferente anchura del etmoides
y del espacio interorbitario. A: Luciobarbus rifensis. B: Luciobarbus maghrebensis. C: Luciobarbus setivimensis. D: Luciobarbus ksibi. E: Población del Moulouya.
|
|
|
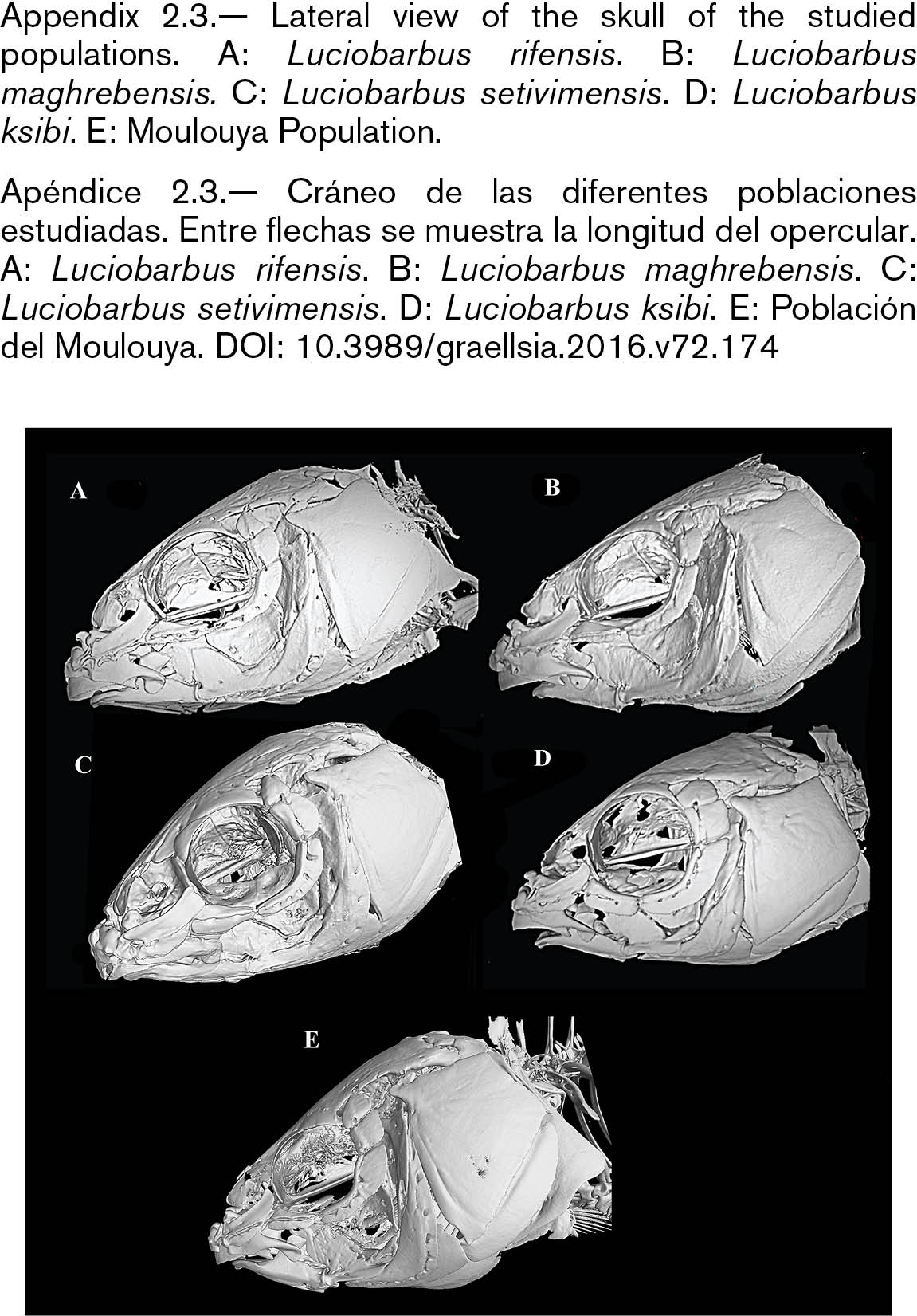 Appendix 2.3.— Lateral view of the skull of the studied populations. A: Luciobarbus rifensis. B: Luciobarbus maghrebensis. C: Luciobarbus setivimensis. D: Luciobarbus ksibi. E: Moulouya Population. Appendix 2.3.— Lateral view of the skull of the studied populations. A: Luciobarbus rifensis. B: Luciobarbus maghrebensis. C: Luciobarbus setivimensis. D: Luciobarbus ksibi. E: Moulouya Population.
Apéndice 2.3.— Cráneo de las diferentes poblaciones estudiadas. Entre flechas se muestra la longitud del opercular. A: Luciobarbus rifensis. B: Luciobarbus maghrebensis. C: Luciobarbus setivimensis. D: Luciobarbus ksibi. E: Población del Moulouya.
|
|
|
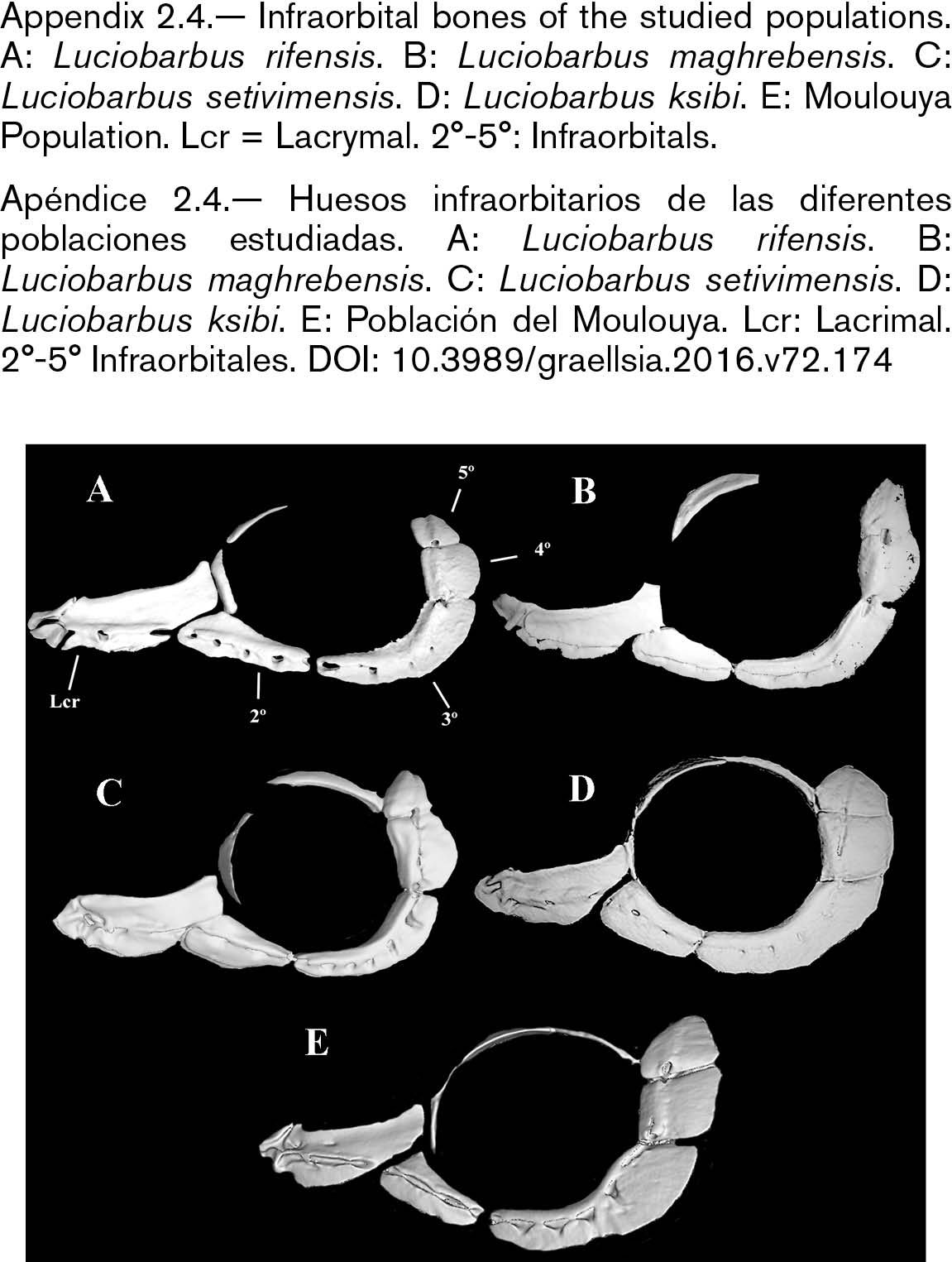 Appendix 2.4.— Infraorbital bones of the studied populations. A: Luciobarbus rifensis. B: Luciobarbus maghrebensis. C: Luciobarbus setivimensis. D: Luciobarbus ksibi. E: Moulouya Population. Lcr = Lacrymal. 2°-5°: Infraorbitals. Appendix 2.4.— Infraorbital bones of the studied populations. A: Luciobarbus rifensis. B: Luciobarbus maghrebensis. C: Luciobarbus setivimensis. D: Luciobarbus ksibi. E: Moulouya Population. Lcr = Lacrymal. 2°-5°: Infraorbitals.
Apéndice 2.4.— Huesos infraorbitarios de las diferentes poblaciones estudiadas. A: Luciobarbus rifensis. B: Luciobarbus maghrebensis. C: Luciobarbus setivimensis. D: Luciobarbus ksibi. E: Población del Moulouya. Lcr: Lacrimal. 2°-5° Infraorbitales.
|
|
|
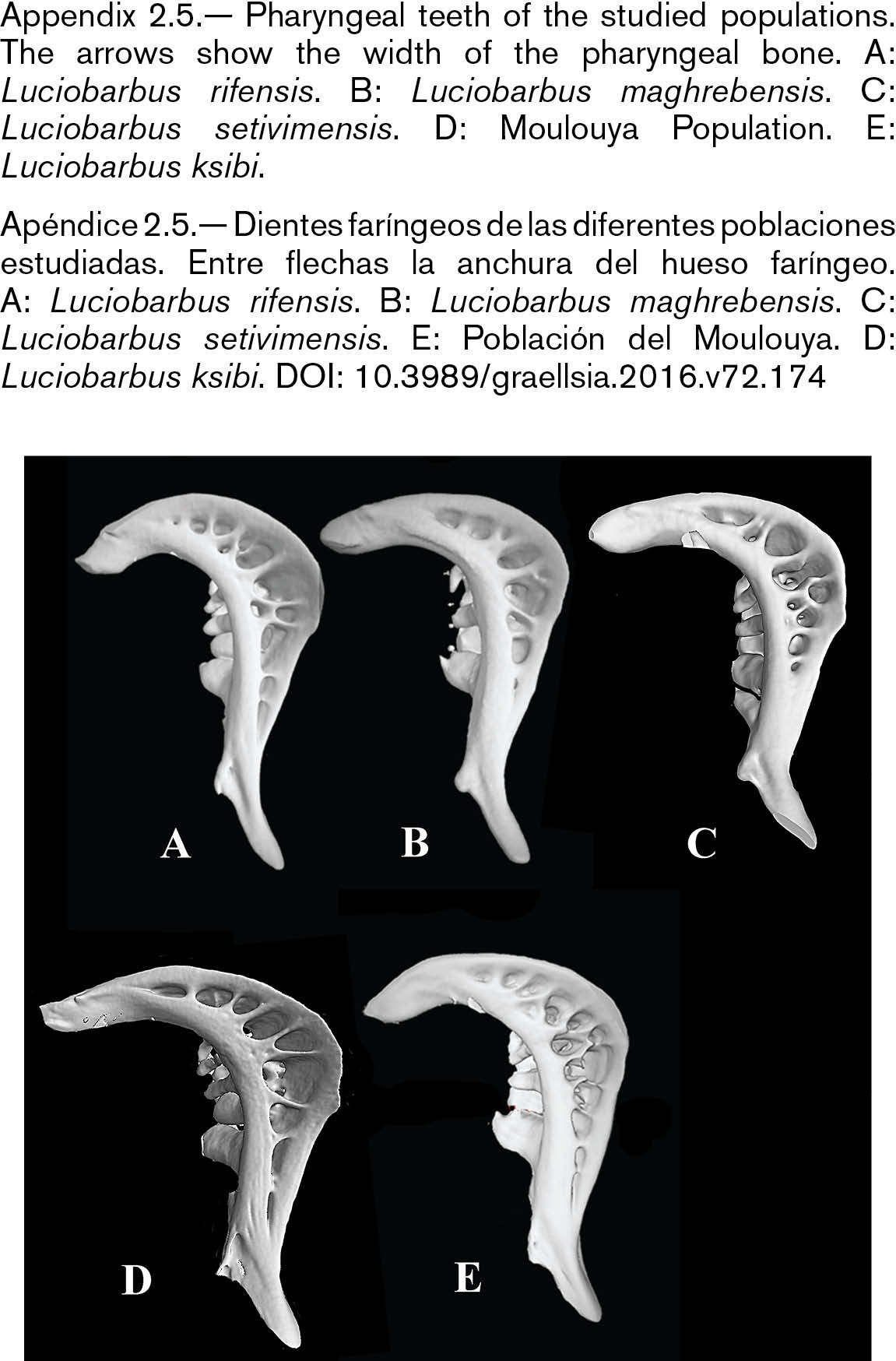 Appendix 2.5.— Pharyngeal teeth of the studied populations. The arrows show the width of the pharyngeal bone. A: Luciobarbus rifensis. B: Luciobarbus maghrebensis. C: Luciobarbus setivimensis. D: Moulouya Population. E: Luciobarbus ksibi. Appendix 2.5.— Pharyngeal teeth of the studied populations. The arrows show the width of the pharyngeal bone. A: Luciobarbus rifensis. B: Luciobarbus maghrebensis. C: Luciobarbus setivimensis. D: Moulouya Population. E: Luciobarbus ksibi.
Apéndice 2.5.— Dientes faríngeos de las diferentes poblaciones estudiadas. Entre flechas la anchura del hueso faríngeo. A: Luciobarbus rifensis. B: Luciobarbus maghrebensis. C: Luciobarbus setivimensis. E: Población del Moulouya. D: Luciobarbus ksibi.
|
|
|
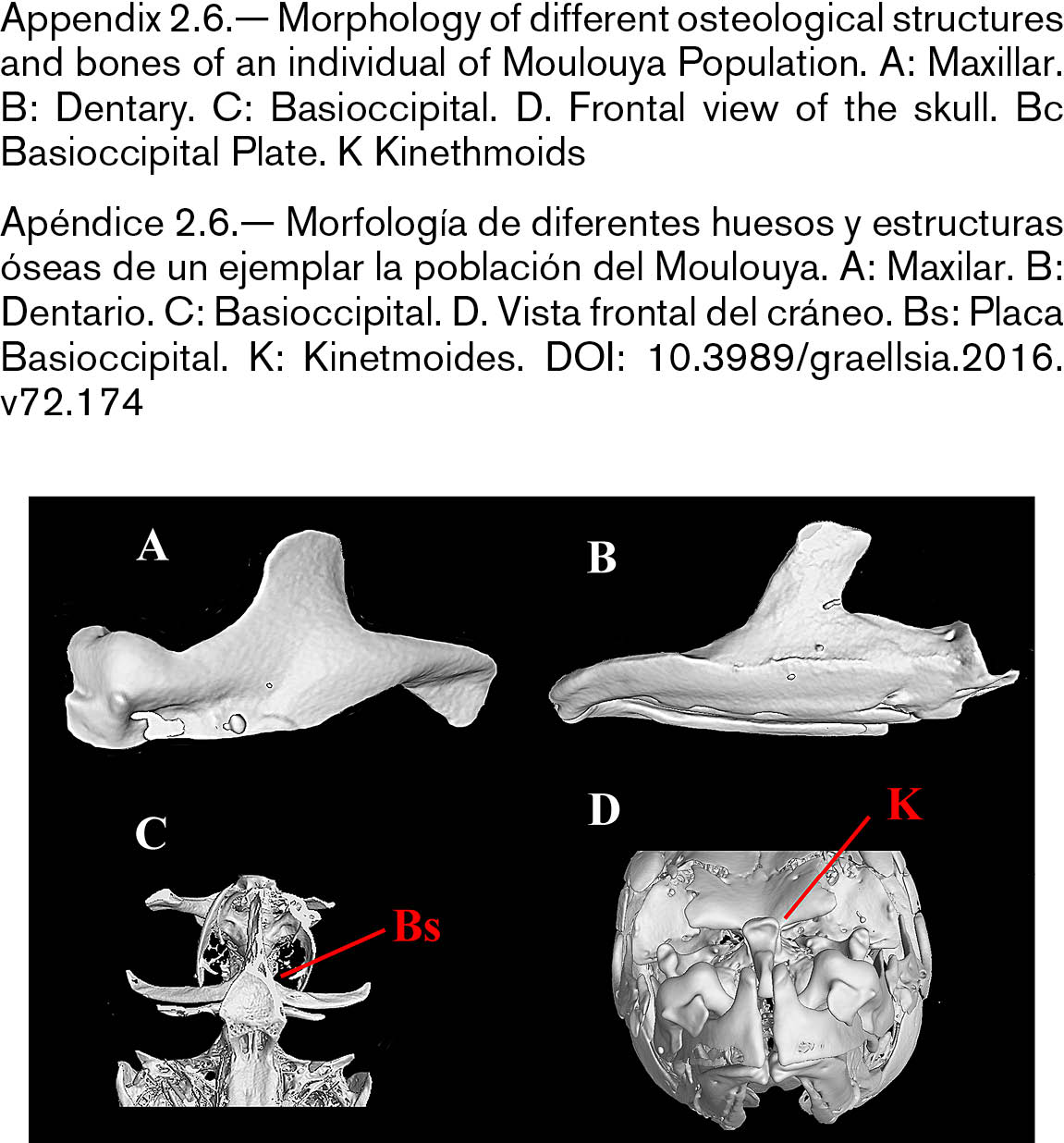 Appendix 2.6.— Morphology of different osteological structures and bones of an individual of Moulouya Population. A: Maxillar. B: Dentary. C: Basioccipital. D. Frontal view of the skull. Bc Basioccipital Plate. K Kinethmoids Appendix 2.6.— Morphology of different osteological structures and bones of an individual of Moulouya Population. A: Maxillar. B: Dentary. C: Basioccipital. D. Frontal view of the skull. Bc Basioccipital Plate. K Kinethmoids
Apéndice 2.6.— Morfología de diferentes huesos y estructuras óseas de un ejemplar la población del Moulouya. A: Maxilar. B: Dentario. C: Basioccipital. D. Vista frontal del cráneo. Bs: Placa Basioccipital. K: Kinetmoides.
|
|
Fig. 1.— Sampling localities of Luciobarbus populations in northern Africa. Moulouya River, Ghafoula (1); Moulouya R., Ksabi (2); Melloulou R., Guercif (3); Zobzite R., Berkine (4); Za R., Guefait (5); Laou R., Derdara (6); Ifrane R., El Hamman (7); Soummam R., Takretz (8); Derna R., Bounoval (9); Chbouka R., El Herri (10); Kasab R., Essaouira (11); Reraia R., Asni (12); El Barred R., Asrire (13).