TAXONOMIC REVIEW OF THE GENUS LUCIOBARBUS HECKEL, 1843 (ACTINOPTERYGII, CYPRINIDAE) FROM NORTHWESTERN MOROCCO WITH THE DESCRIPTION OF THREE NEW SPECIES
Miriam Casal-Lopez1, Silvia Perea1, Ahmed Yahyaoui2 & Ignacio Doadrio1*
1Biodiversity and Evolutionary Group. National Museum of Natural Sciences. CSIC. C/ José Gutiérrez Abascal, 2. 28006. Madrid.
Spain
MC-L: urn:lsid:zoobank.org:author:84DEBB1B-A34C-412A-B03B-C3492A4E59BE
SP: urn:lsid:zoobank.org:author:75C3112F-5BE9-4512-A9DB-DDA2A10497C7
ID: urn:lsid:zoobank.org:author:1514FE9E-2AA2-46D1-BB43-51F5E8EF1566
2Laboratory of Zoology and General Biology, Faculty of Sciences, Mohammed V University. B.P. 1014. Rabat. Morocco
urn:lsid:zoobank.org:author:EA344560-E0FA-4D5D-A51C-23771D124D4B
* Corresponding author: doadrio@mncn.csic.es
| |
ABSTRACT
The genus Luciobarbus in Morocco presents high diversification, in contrast to the generally impoverished freshwater fish fauna from North Africa.
Within Morocco the northern area is one of the least studied territories, due to both its historical background and the limited
accessibility of many regions. Previous phylogenetic studies identified Luciobarbus populations that are morphologically and genetically differentiated, to the same extent as others already recognized as separate
species. The aim of this work is to describe these populations as distinct species, based on morphological, meristic, and
genetic traits.
urn:lsid:zoobank.org:pub:8FC4B423-104C-4097-A468-ED3D2664A15A
Key words: Cyprinidae;
Luciobarbus;
taxonomic review;
new species;
Morocco.
|
| |
RESUMEN
Revisión taxonómica del género Luciobarbus Heckel, 1843 (Actinopterygii, Cyprinidae) del noroeste de Marruecos con la descripción de tres nuevas especies
En Marruecos el género Luciobarbus está altamente diversificado, en comparación con la empobrecida fauna de peces de agua dulce del norte de África. Dentro
de Marruecos la región norte es una de las áreas menos estudiadas, por motivos históricos y también por la poca accesibilidad
en algunas de sus regiones. Los estudios filogenéticos previos para esta área han señalado la existencia de poblaciones pertenecientes
al género Luciobarbus tan diferenciadas morfológica y genéticamente como otras que ya habían sido reconocidas como diferentes especies. En este
trabajo describimos estas poblaciones como diferentes taxa, en base a caracteres morfológicos, merísticos y genéticos.
Palabras clave: Cyprinidae;
Luciobarbus;
revisión taxonómica;
nuevas especies;
Marruecos.
|
IntroductionTOP
The genus Barbus sensu lato includes more than 800 species and it is one of the genera with the highest number of species among vertebrates. It is a
polyphyletic assemblage with different genetic and morphologic features. In fact, in recent years, Barbus phylogenetic studies have led to its division into different genera (Karaman, 1971; Machordom & Doadrio, 2001a; Yang et al., 2015).
European and North African species previously included in the genus Barbus Cuvier & Cloquet, 1843 are presently included in the genera Barbus and Luciobarbus Heckel, 1843 (Kottelat & Freyhof, 2007). The genus Luciobarbus was initially assigned to the Asian species Luciobarbus esocinus Heckel, 1843 but was long considered as a synonym of Barbus Cuvier & Cloquet, 1843. Notwithstanding, a phylogenetic study using morphological characters revealed that most limnophilic
circum-Mediterranean species occurring in northern Africa, the Mediterranean peninsulas, and the Near East were monophyletic
(Doadrio, 1990). This monophyletic clade was included within the subgenus Luciobarbus (Doadrio, 1990), which with the advent of molecular studies, was better defined and recognized at the generic level (Machordom & Doadrio,
2001b; Tsigenopoulos et al., 2003; Griffiths et al., 2004; Kottelat & Freyhof, 2007; Yang et al., 2015).
Molecular studies of the genus Luciobarbus have, so far, been based on isoenzyme analysis (Machordom et al., 1995, 1998; Doadrio et al., 1998) and sequencing of mitochondrial genes (Zardoya & Doadrio, 1999; Zardoya et al., 1999; Machordom & Doadrio 2001b; Doadrio et al., 2002; Tsigenopoulos et al., 2003). The sequencing of nuclear genes has been constrained by the tetraploid condition of the species of this genus (Yang et al., 2015).
Results of previous molecular studies have revealed a strong Luciobarbus population structure in North Africa (Berrebi et al., 1995; Doadrio et al., 1998; Machordom et al., 1998; Machordom & Doadrio, 2001b). Sixteen populations, genetically isolated during the late Miocene and Pliocene, were recognized in North Africa, some of
them assigned to different species (Machordom & Doadrio, 2001b). Within North African populations, those of northwestern Morocco, inhabiting areas ranging from the basins of the Laou River
(on the Mediterraenan slope in the North) to the Kasab River (on the Atlantic slope in the southwest), have been clustered
together by genetic studies (Machordom & Doadrio, 2001b; Geiger et al., 2014). However, there are few taxonomic studies focusing on Luciobarbus populations of northwestern Morocco, due to its traditional isolation and limited accessibility (Almaça, 1966, 1968, 1970; Doadrio, 1990). For these reasons northern Morocco was ignored, when taxonomic African ichthyological studies were conducted by the French
Geographical Society, in the early twentieth century.
Some Luciobarbus populations, mainly from Laou, Grou and Sebou Basins, belonging to northwestern Morocco present high genetic differentiation
in several molecular markers, such as isozymes and the mitochondrial cytochrome b gene, compared to other populations of the genus and constitute a monophyletic group (Machordom et al., 1998; Machordom & Doadrio, 2001b; Geiger et al., 2014). Nonetheless, only morphological works have been carried out for the Sebou Basin (Almaça, 1966, 1968, 1970; Doadrio, 1990). The aim of our study was to extend the molecular and morphological works to other populations from northwestern Morocco.
Thus, our goal was to clarify, with a more complete sampling, the taxonomy of Luciobarbus in northwestern Morocco with an integrative approach and unravel whether the diversity found corresponds to new taxa that
may not have been formally described so far. In this case, we will formally describe these new taxa.
Material and MethodsTOP
MORPHOLOGICAL ANALYSESTOP
The taxonomy of the population of northwestern Morocco was based on 47 specimens from Laou Basin, 43 specimens from Loukos
Basin, 22 specimens from Hachef Basin, 55 specimens from Sebou Basin, 33 specimens from Bou Regreg Basin and 23 specimens
from Kasab Basin (Fig. 1, Table 1).
|
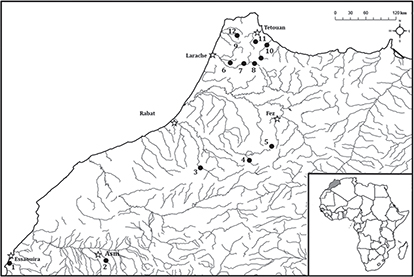 Fig. 1.— Geographic distribution of Luciobarbus spp. and sampling localities. The numbers on map correspond to localities in Table 1. Fig. 1.— Geographic distribution of Luciobarbus spp. and sampling localities. The numbers on map correspond to localities in Table 1.
Fig. 1.— Distribución geográfica de Luciobarbus spp. y localidades muestreadas. La correspondencia entre números y localidades se puede ver en la Tabla 1.
|
|
Table 1.— Sampling localities for Luciobarbus spp. Code is the number on the phylogenetic tree. G= Genetic, M= Morphometry.
Tabla 1.— Localidades muestreadas de las especies
de Luciobarbus estudiadas. El código es el número que aparece en el árbol filogenético. G= análisis genético. M= análisis morfométrico.
| Species |
River |
Locality |
Basin |
Analyses |
Code |
GenBank |
Map |
| L. ksibi |
Kasab |
Essaouira |
Kasab |
G,M |
K6-K10 |
KT003951-55 |
1 |
| L. ksibi |
Reraia |
Asni |
Tensift |
G |
K1-K5 |
KT003956-60 |
2 |
| L. sp1 |
Tizguit |
Ifrane |
Sebou |
M |
|
|
4 |
| L. sp1 |
Ifrane |
Ouad Ifrane |
Sebou |
G,M |
S1-S6 |
KT003941-45 |
5 |
| L. sp2 |
Tattofte |
Ouled Soltane |
Loukos |
M |
|
|
6 |
| L. sp2 |
Loukos |
Mouries |
Loukos |
M |
|
|
7 |
| L. sp2 |
Loukos |
Souk Had, Laghdir |
Loukos |
G |
RK1-RK5 |
KT003936-40 |
8 |
| L. sp2 |
Hachef |
Dar Chaoui |
Hachef |
G,M |
RH1-RH5 |
KT003931-35 |
9 |
| L. sp2 |
Laou |
Derdara |
Laou |
G,M |
RL1-RL5 |
KT003926-30 |
10 |
| L. sp2 |
Laou |
Beni Ferten |
Laou |
M |
|
|
11 |
| L. sp3 |
Bou Regreg |
Sebt Ait Rahhou |
Grou |
G |
B1-B5 |
KT003946-50 |
3 |
| L. capito |
Terek |
Kizlyar |
Terek (Russia) |
G |
L. capito |
AF045975 |
|
The material studied comsisted of the following locations and number of specimens: 25 adult specimens from the Laou River,
Laou Basin, Derdara, (35.118986, -5.288900), Morocco (Voucher numbers: MNCN 290.639-290.652, 290.655, 290.657-663, 290.665-667);
22 specimens from the Laou River, Laou Basin, Beni Ferten (35.353254, -5.184840), Morocco (Voucher numbers: MNCN 284.939-940,
284.942-945, 284.947-284.948, 284.950-951, 284.953-964); 43 specimens from the Loukos River, Loukos Basin, Souk Had, Laghdir
(35.02624,-5.404660), Morocco (Voucher numbers: MNCN: 280.162-163, 280.165, 280.168, 280.170, 280.172-174, 280.176-181, 280.183,
280.185-186, 290.671-696); 22 specimens from the Hachef River, Hachef Basin, Dar Chaoui (35.526763,-5.713771), Morocco (MNCN
290.707-714; 290.716-722; 290.725-731); 32 specimens from the Ifrane River, Sebou Basin, Ouad Ifrane, (33.296957, -5.492639)
Morocco (voucher numbers: MNCN 279.711-729, 290.731, 279.733-744); 23 specimens from the Tizguit River, Sebou Basin, Ifrane
(33.549241, -5.097144), Morocco (Voucher number: MNCN 71675-697); and 33 specimens from the Grou River, Bou Regreg Basin,
Sebt Ait Rahhou (33.164678, -6.366963), Morocco (Voucher numbers: MNCN 71725-746, 71918-28). For comparative purposes, we
analyzed 23 specimens of Luciobarbus ksibi Boulenger 1905 from species type locality in the Kasab River, Kasab Basin, Essaouira (31.465857,-9.759850) Morocco (Voucher
numbers: MNCN 105.460-65, 105.469, 234.925-29, 280.483-485, 71220-21, 71223-26, 71230, 290.670). All sampling sites (Fig. 1) presented similar riverine morphology, with clear water and fast current and gravel bottom, with the exception of the Kasab
River, in Essaouira, which showed a more marked seasonal regime and poorer water conditions.
Twenty-three morphometric measurements (in mm) and nine meristic variables were recorded from digital photographs using TpsDig
v.1.4 (Rohlf, 2003). The following abbreviations were used for morphometric and meristic characters: A, anal fin rays; AFH, anal fin height;
AFL, anal fin length; APL, anal peduncle length; BL1, first barbel length; BL2, second barbel length; BD, body depth; LBD,
lowest body depth; C, central caudal fin rays; CFL, caudal fin length; CPL, caudal peduncle length; D, dorsal fin rays, DFL
dorsal fin length; DFH dorsal fin height; ED, eye diameter; HL, head length; LL lateral line scales; P, pectoral fin rays;
PFL, pectoral fin length; PrAD, pre-anal distance; PrDD, pre-dorsal distance; PrOL, pre-orbital length; PrPD, pre-pectoral
distance; PrVD, pre-ventral distance; PsOL, postorbital length; PVL, pectoral-ventral length; RSA, scale rows above lateral
line; RSB scale rows below lateral line; SL, standard length; V, ventral fin rays; VFL, ventral fin length; VE, Number of
vertebrae. The number of vertebrae was obtained by direct counting on X-ray images of individuals from all populations sampled.
After constructing the measurement matrix, Burnaby’s method was used to correct size effect (Burnaby, 1966; Rohlf & Bookstein, 1987). All analyses were conducted with the corrected matrix. Morphometric and meristic characters were analyzed independently.
A two-way analysis of variance (ANOVA) comparing morphometric characters was conducted to test for sexual dimorphism and variation
among populations. To identify the variables that contributed most to the variation between populations, a principal components
analysis (PCA) was performed using the covariance matrix for morphometric characters.
MOLECULAR ANALYSESTOP
For the molecular approach, we analyzed samples corresponding to individuals of Luciobarbus spp. from Sebou, Laou, Hachef, Loukos and Bou Regreg basins; and Luciobarbus ksibi from the Kasab Basin (Table 1). The species Luciobarbus capito (Güldenstädt, 1773) was selected as outgroup based on previous phylogenetic analyses (Zardoya & Doadrio, 1999). Total genomic DNA was extracted from fin-clip tissue using the commercial kit Biosprint 15 for tissue and blood (Qiagen).
For each specimen, the complete region (1140bp) of the mitochondrial cytochrome b (cytb) was amplified. Primers and protocols used for PCR for cytb followed Machordom & Doadrio (2001b). After checking PCR products on 1% agarose gels, they were purified by ExoSAP-IT™ (USB) and directly sequenced on MACROGEN
service using a 3730XL DNA sequencer. All sequences were deposited in the GenBank database (Accession Numbers: KT003926-KT003960).
PHYLOGENETIC ANALYSESTOP
Phylogenetic analyses were performed using Bayesian inference (BI) implemented in MrBayes v.3.2 (Ronquist et al., 2012). The Akaike Information Criterion (Akaike, 1973) implemented in jModeltest (Posada, 2008) was used to determine the evolutionary model that best fit the data. In this case TrN+I model was selected (R(a) [AC] =1.0000,
R(b) [AG] =92.1342, R(c) [AT] =1.0000, R(d) [CG] =1.0000, R(e) [CT] =20.3361, R(f) [GT] =1.0000, p-inv =0.7900). BI was performed
using two independent runs of four Markov Montecarlo coupled chains (MCMC) of 106 generations each, to estimate the posterior probability distribution. Topologies were sampled every 100 generations, and
majority-rule consensus tree was estimated after discarding the first 10% of generations. Robustness of clades was assessed
using Bayesian posterior probabilities. The average genetic distances among Luciobarbus populations were calculated for each gene using MEGA package v.6.0 (Tamura et al., 2013) according to the uncorrected-p distances.
Results and DiscussionTOP
COMPARISON OF MORPHOLOGY AMONG POPULATIONSTOP
Two-way analysis of variance (ANOVA), testing for sexual dimorphism and differentiation among populations, showed significant
differences (p<0.05) for the variables standard length, postorbital length, and anal fin size (Table 2). In absolute values all variables were greater in females, but proportionally to the standard length postorbital length
was longer in males (Table 3). A non-biological interpretation could explain the differences in postorbital length by sex, therefore we performed a test
of Mann-Whitney-Wilcoxon at p<0.05, with postorbital length and sex as variables, for each population independently. The null
hypothesis for identical postorbital length between males and females for each population was not rejected in all populations
(Sebou Basin z=−0.810; Bou Regreg Basin z=−0.868; Kasab Basin z=−1.692; Loukos Basin z=−0.480; Laou Basin z=−0.343; Hachef Basin z=−0.032). Therefore, the differences in postorbital length were not explained by sex and were an effect of population differences.
Table 2.— Two-way analysis of variance (ANOVA) for sexual dimorphism, population variation, and their interaction. Significant
differences p<0.05 (*); p<0.01 (**). N=163 males and n=60 females. Acronyms are defined in the Material and Methods.
Tabla 2.— Análisis de la varianza (ANOVA) de dos vías para dimorfismo sexual, variación poblacional y su interacción. Diferencias
significativas p<0,05 (*); p<0,01 (**). N=163 machos and N=60 hembras. Las abreviaturas se describen en el epígrafe de Material
y Métodos.
| Variables |
Sexual dimorphism (f/p-value) |
Population Variation (f/p-value) |
Sex/pop variation (f/p-value) |
| SL |
4.51/* |
53.38/** |
2.37/ |
| HL |
3.06/ |
26.74/** |
1.94/ |
| PrOL |
1.45/ |
13.36/** |
2.08/ |
| ED |
0.22/ |
11.32/** |
0.57/ |
| PsOL |
22.32/** |
469.5/** |
7.58/** |
| B1L |
3.45/ |
10.09/** |
2.17/ |
| B2L |
1/ |
21.07/** |
0.19/ |
| PrDD |
1.66/ |
78.49/** |
2.14/ |
| PrPD |
0.01/ |
44.54/** |
4/** |
| PrVD |
3.18/ |
68.28/** |
3.55/* |
| PrAD |
1.75/ |
56.71/** |
3.5/* |
| CPL |
0.96/ |
11.14/** |
1.92/ |
| APL |
0.51/ |
3.68/* |
4.39/** |
| PVL |
0.01/ |
12.71/** |
0.51/ |
| BD |
3.13/ |
9.64/** |
1.34/ |
| BLD |
0.001/ |
6.93/** |
1.61/ |
| DFL |
2.04/ |
2.9/ |
0.137/ |
| DFH |
3.31/ |
27.42/** |
2.32/ |
| PFL |
0.10/ |
23.08/* |
1.8/ |
| VFL |
2.42/ |
14.26 /** |
0.19/ |
| AFL |
4.26/* |
16.61/ |
2.37/ |
| AFH |
11.46/** |
29.88/** |
2.06/ |
| CFL |
0.87/ |
16.08/** |
1.41/ |
Table 3.— Morphometric variables showing significant sexual dimorphism (p<0.05). Values are mean (minimum-maximum). Acronyms
are defined in the Material and Methods.
Tabla 3.— Variables morfométricas que muestran dimorfismo sexual significativo (p<0.05).
Los valores son medias y entre paréntesis valores máximos y mínimos. Las abreviaturas están descritas en el epígrafe de Material
y Métodos.
| Variables (mm) |
Males n=163 |
Females n=60 |
| SL |
106.2 (53.1-232.8-75) |
157.7 (82.1-240.1) |
| PsOL |
13.5 (6.6-30.8) |
18.9 (9.6-29.6) |
| AFL |
8.3 (3.74-17.8) |
12.3 (6.2-19.3) |
| AFH |
18.3 (10-41.6) |
27.5 (15-44.8) |
Females had proportionally higher and longer anal fins (Table 3). Sex differences in the size of anal fins have been found in other Luciobarbus species, and are probably associated with the female’s use of the anal fin to excavate nests in the riverbed (Banarescu & Bogutskaya, 2003). Nuptial tubercles were present in the snout and head of males, particularly around the preorbital region.
To deal with the presence of sexual dimorphism in anal fin size, we removed AFL and AFH from posterior analyses. Most morphometric
variables showed significant differences between populations in the two-way ANOVA analysis (Table 2). An analysis of body proportions based on Kruskal-Wallis and Mann-Whitney post hoc comparisons, was used to detect differences in body shape that can masked if only linear untransformed measurements are taken
into account (Appendix 1). We grouped the populations from the Rif Mountains (Laou, Loukos, and Hachef Basins) based on mitochondrial
DNA analysis and previous genetic studies (Machordom et al., 1998, Machordom & Doadrio, 2001b; Geiger et al., 2014). The populations from Sebou Basin showed a shorter predorsal distance and a longer caudal peduncle than the other Luciobarbus populations studied, due to the more anterior position of the dorsal fin (Fig. 2, Table 4).
|
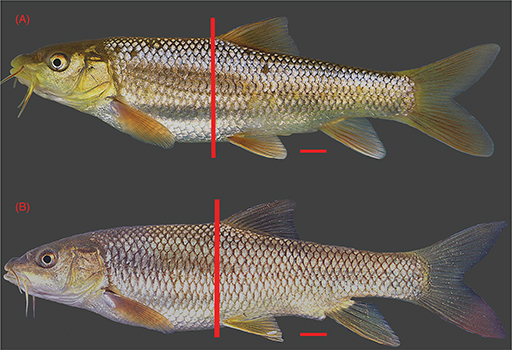 Fig. 2.— Comparison of two females of similar standard length from (A) Sebou Basin and (B) Laou Basin (Rifian population) showing
differing position of the dorsal fin with respect to ventral fin insertion. Fig. 2.— Comparison of two females of similar standard length from (A) Sebou Basin and (B) Laou Basin (Rifian population) showing
differing position of the dorsal fin with respect to ventral fin insertion.
Fig. 2.— Comparación entre dos hembras de similar
longitud estándar de las cuencas del Sebou (A) y del río Laou (población del Rif) (B) mostrando la diferente posición de la
aleta dorsal con respecto al origen de la aleta ventral.
|
|
Table 4.— Ratios of morphometric variables and scale count. Values are mean (minimum and maximum). Acronyms are defined in
the Material and Methods.
Tabla 4.— Valores de la media, y entre paréntesis valores mínimos y máximos, para diferentes proporciones
de las variables morfométricas y número escamas. Las abreviaturas están descritas en el epígrafe de Material y Métodos.
| Measurements |
Rif populations (n=112) |
Sebou (n=55) |
Bou Regreg (n=33) |
Luciobarbus ksibi (n=23)
|
| SL/PrDD |
1.87 (1.79-1.95) |
1.93 (1.79-2.03) |
1.85 (1.78-1.96) |
1.85 (1.73-1.98) |
| SL/CPL |
2.81 (2.56-3.06) |
2.7 (2.54-2.91) |
2.79 (2.63-2.91) |
2.82 (2.63-3.17) |
| HL/PsOL |
2.21 (1.65-2.50) |
1.61 (1.29-1.81) |
2.04 (2.47-3.41) |
2.23 (2.03-2.57) |
| HL/PrOL |
2.74 (2.35-3.23) |
2.82 (2.48-3.49) |
2.97 (2.47-3.5) |
2.78 (2.4-3.1) |
| SL/BLD |
9.11 (7.48-10.92) |
8.72 (7.57-10) |
8.38 (8.06-8.7) |
7.8 (7.36-8.27) |
| CPL/BLD |
3.24 (2.46-3.83) |
3.24 (2.67-3.75) |
3 (2.81-3.26) |
2.78 (2.6-2.99) |
| APL/BLD |
1.64 (1.25-2.07) |
1.58 (1.37-1.83) |
1.55 (1.39-1.67) |
1.5 (1.29-1.87) |
| HL/L1B |
4.16 (2.78-6.87) |
3.96 (3.03-5.27) |
4.11 (3.8-4.5) |
3.5 (2.93-4.05) |
| HL/L2B |
3.27 (2.48-5) |
3.1 (2.4-4.74) |
3.29 (3.05-3.52) |
2.65 (2.26-3.25) |
| LL |
43.9 (46-42) |
43.7 (41-47) |
46.5 (45-50) |
43.7 (41-47) |
| RSA |
8.8 (8.5-9.5) |
7.5 (7.5-8.5) |
9.6 (8.5-10.5) |
8.3 (6.5-8.5) |
| RSB |
4.7 (3.5-5.5) |
5.5 (4.5-6.5) |
6.74 (6.5-7.5) |
4.98 (4.5-5.5) |
The Sebou population exhibited greater postorbital length with respect to the other studied populations, and therefore the
ratio SL/PsOL in the Sebou population was the lowest (Table 4). The caudal peduncle was higher in Luciobarbus ksibi (Kasab population) than in the other studied populations, and the ratios CPL/CPI and APL/BLD, were the lowest-. Barbels were
longest in Luciobarbus ksibi. Preorbital length was significantly shorter in the Bou Regreg population compared to the other populations studied.
Lateral line scales were more numerous in the Bou Regreg population than in other populations. Scales numbers on the superior
transverse line were higher in Bou Regreg and Rifian populations than in L. ksibi and Sebou populations, while the number of scales on the inferior transverse line was lower in Rifian populations and L. ksibi (Table 4).
The principal component analysis divided the populations into three groups corresponding to Luciobarbus ksibi and populations of the Rifian and the Sebou Basins. The population of the Bou Regreg Basin was included in the morphometric
variability of Rifian populations (Fig. 3). Nevertheless, the Bou Regreg population could be discriminated from the other studied populations by the number of scales
on the body (Fig. 4).
|
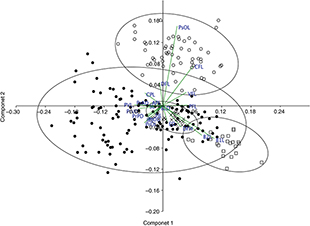 Fig. 3.— Variables that most contributed to the PCA analysis. Dots, Rifian populations. Squares, Luciobarbus ksibi from Kasab Basin. X, Population from Bou Regreg Basin. Circles, population from Sebou Basin. Abbreviations are defined in
Materials and Methods. Fig. 3.— Variables that most contributed to the PCA analysis. Dots, Rifian populations. Squares, Luciobarbus ksibi from Kasab Basin. X, Population from Bou Regreg Basin. Circles, population from Sebou Basin. Abbreviations are defined in
Materials and Methods.
Fig. 3.— Variables que más contribuyen al ordenamiento en el PCA. Puntos poblaciones del Rif. Cuadrados
Luciobarbus ksibi de la cuenca del Kasab. Equis población de la cuenca del Bou Regreg. Círculos población de la cuenca del Sebou. Las abreviaturas
están descritas en el epígrafe de Material y Métodos.
|
|
|
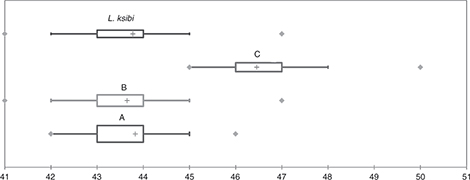 Fig. 4.— Box-plots of lateral line scale numbers. A, Rifian population; B, Sebou Basin population; C, Bou-Regreg Basin population. Fig. 4.— Box-plots of lateral line scale numbers. A, Rifian population; B, Sebou Basin population; C, Bou-Regreg Basin population.
Fig. 4.— Box-plots para el número de escamas en la línea lateral. A, Población del Rif; B, Población de la Cuenca del Sebou; C,
Población de la Cuenca del Bou Regreg.
|
|
The eigenvalues of the two first principal components, with the Burnaby-corrected matrix, explained most of the variance (Table 5). The highest values for eigenvectors in both males and females, and, consequently, the variables that contributed most to
the ordination in the PCA were: postorbital and preorbital length, caudal and dorsal fin size, and barbel length (Table 5).
Table 5.— Eigenvalues and eigenvectors for the first three principal components (PC1-PC3) of 21 morphometric variables for
all Luciobarbus populations. Acronyms are defined in the Material and Methods. In bold, variables with the highest eigenvectors for each
PC.
Tabla 5.— Eigenvalores y eigenvectores para los tres primeros componentes principales (PC1-PC3) de 21 variables morfométricas
para todas las poblaciones del género Luciobarbus. Las abreviaturas están descritas en el epígrafe de Material y Métodos. En negrita, variables con los eigenvectores más altos
para cada CP.
| Variables |
PCI |
PCII |
PCIII |
| Eigenvalue |
0.0073 |
0.0044 |
0.0029 |
| % variance |
29.1 |
17.56 |
11.48 |
| Eigenvectors |
| SL |
−0.1416 |
−0.0053 |
0.0137 |
| PrDD |
−0.1419 |
−0.0904 |
0.0254 |
| PrPD |
−0.1741 |
−0.0915 |
−0.0420 |
| PrVD |
−0.2401 |
−0.0236 |
−0.0667 |
| PrAD |
−0.1961 |
0.0014 |
−0.0377 |
| PVL |
−0.2772 |
0.0095 |
−0.0804 |
| CPL |
−0.1750 |
0.1154 |
0.0243 |
| APL |
−0.1185 |
0.0260 |
0.1110 |
| BD |
−0.1550 |
−0.1024 |
−0.0021 |
| BLD |
−0.0296 |
−0.0078 |
−0.0763 |
| HL |
−0.0841 |
−0.0731 |
−0.0450 |
| PrOL |
−0.1817 |
−0.1536 |
−0.0761 |
| ED |
0.0442 |
−0.1513 |
−0.1591 |
| PsOL |
0.1385 |
0.7205 |
−0.3674 |
| B1L |
0.5001 |
−0.2952 |
−0.4918 |
| B2L |
0.3794 |
−0.2658 |
−0.1933 |
| PFL |
0.2412 |
0.0068 |
0.2772 |
| VFL |
0.2282 |
0.1219 |
0.1780 |
| DFL |
−0.0322 |
−0.2115 |
−0.0283 |
| DFH |
0.1795 |
0.3645 |
0.4958 |
| CFL |
0.2966 |
0.2586 |
−0.4046 |
OSTEOLOGICAL FEATURESTOP
The hardness of the last single ray of the dorsal fin (DFR) and its number of denticulations frequently has been used in the
taxonomy of Luciobarbus (Almaça, 1970). As Doadrio (1990) pointed out, there is ontogenetic variability in DFR, and comparison of the characteristics of DFR among species, for taxonomic
purposes, must be done using adult specimens (Appendix 2-1). We found L. ksibi presents strong denticulations but only over 2/3 of the DFR length. Denticulations of the DFR in specimens of the Bou Regreg
population were deep and the length of these denticulations was greater than the width of the ray. Dorsal fin rays of the
Rifian and Sebou basins populations were similar, slightly stronger in Rifian populations than in the Sebou population.
The skulls of L. ksibi and Sebou populations are shorter and wider than those of the Bou Regreg and Rifian populations. The ethmoid bone in the
Sebou population is narrower than in other studied populations, especially pronounced compared to the Rifian population (Append.
2-2). In the skull lateral view, the opercular bone appeared longer in the Sebou population than in other populations (Append. 2-3). Infraorbital bones are larger in L. ksibi than in other populations, and the second infraorbital bone is longer in Rifian than in other populations and usually exhibits
four pores (Append. 2-4). The pharyngeal bone is thinner in the Bou Regreg populations and wider in L. ksibi. The dorsal branch of the pharyngeal bone forms a closed angle with respect to inferior branch in Rifian population and L. ksibi (Append. 2-5).
MOLECULAR DATATOP
Phylogenetic analyses based on the cytb gene supported four main clades in the tree, corresponding to the populations of the Rif, Sebou, and Bou Regreg Basins and
Luciobarbus ksibi (Fig. 5). The genetic distances found among populations are presented in Table 6. Genetic distances within Sebou, Bou Regreg, and Rifian populations and L. ksibi ranged between 0-0.6% but between these populations were 1.7-3.9%. The minimum value was found between L. ksibi and Bou Regreg populations (Table 6).
|
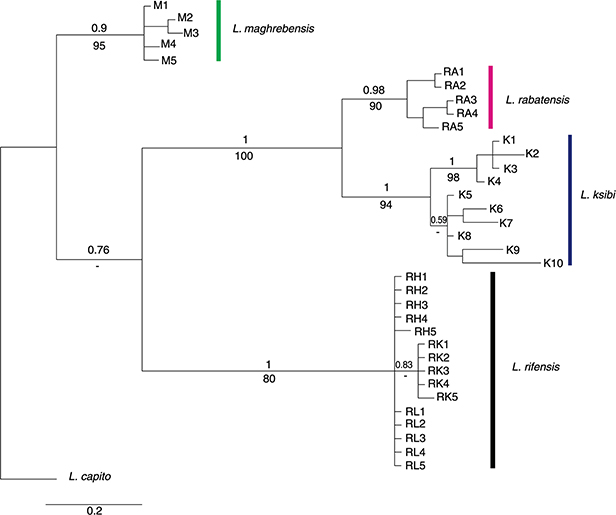 Fig. 5.— Phylogenetic tree rendered by Bayesian Inference of the cytochrome b gene. Numbers on branches indicate posterior probability values. Abbreviations of localities are defined in Table 1. Fig. 5.— Phylogenetic tree rendered by Bayesian Inference of the cytochrome b gene. Numbers on branches indicate posterior probability values. Abbreviations of localities are defined in Table 1.
Fig. 5.— Árbol filogenético obtenido por Inferencia Bayesiana para el gen citocromo b. Los números en las ramas indican los valores de probabilidad posterior. Las abreviaturas de las localidades se muestran
en la Tabla 1.
|
|
Table 6.— Genetic distances for complete mitochondrial cytb gene. Uncorrected genetic distances between populations are presented below diagonal. In the diagonal uncorrected genetic
distances within populations are shown.
Tabla 6.— Distancias genéticas. Por debajo de la diagonal distancias genéticas no corregidas
entre poblaciones para el gen mitocondrial cytb. En la diagonal distancias genéticas no corregidas dentro de poblaciones.
|
Laou (Rif) |
Hachef (Rif) |
Loukos (Rif) |
Sebou |
Bou Regreg |
| Hachef |
0.0 |
|
|
|
|
| Loukos |
0.2 |
0.2 |
|
|
|
| Sebou |
3.2 |
3.2 |
3.2 |
|
|
| Bou Regreg |
3.9 |
3.9 |
3.9 |
3.1 |
|
| L. ksibi |
3.8 |
3.8 |
3.9 |
3.7 |
1.7 |
The distances are of similar range as those between well recognized species of cyprinid fishes (Doadrio et al., 2002, 2007a, 2007b; Doadrio & Carmona, 2003, 2006; Doadrio & Madeira, 2004; Robalo et al., 2005; Doadrio & Elvira, 2007; Domínguez-Domínguez et al., 2007, 2009). These results confirm the differences found with allozyme analyses (Machordom et al., 1998). Allozyme studies found 23 polymorphic loci, four of which (MPI, GPI-5, IDHP-2, CK) were diagnostic, with a probability
criterion of 99% of correct assignment to Rifian populations with respect to the Sebou Basin. Between the Sebou Basin (Ifrane
population) and Grou Basin three diagnostic loci were found (GPI-2*, GPI-5* and PGDH-1). The population of the Grou River
(Bou Regreg Basin) exhibited a unique diagnostic locus (GPI-2* 118) with respect to all Luciobarbus populations (Machordom et al., 1998). Populations from Sebou, Rif and Bou Regreg basins were identified as different taxa by other authors based on the molecular
differences found, but were not formally described as new so far (Machordom et al., 1998).
TAXONOMY OF LUCIOBARBUS LABIOSUS (PELLEGRIN, 1920)TOP
Initially the population from Sebou Basin in Morocco was considered to belong to Barbus setivimensis Valenciennes, 1842, which was originally described for populations of the Setif Basin in Algeria (Pellegrin, 1920). Within the Sebou population of B. setivimensis, Pellegrin (1920) found specimens with well-developed lips that this author described as a variety, B. setivimensis var. labiosa Pellegrin, 1920. This development of the lips in some specimens of the former genus Barbus sensu lato, currently within Luciobarbus, is common, and Pellegrin (1922) described another variety as Barbus massaensis var. labiosa Pellegrin, 1922. As Almaça (1968, 1970) stated, Pellegrin (1920) did not describe the var. labiosa as a subspecific category or geographic form because he found typical specimens of B. setivimensis and some specimens with more developed lips in the same locality. For this reason Article 45.6.4 of the International Code of Zoological Nomenclature is not applicable:
“45.6.4. it is subspecific if first published before 1961 and its author expressly used one of the terms “variety” or “form”
(including use of the terms “var.”, “forma”, “v.” and “f.”), unless its author also expressly gave it infrasubspecific rank,
or the content of the work unambiguously reveals that the name was proposed for an infrasubspecific entity, in which case
it is infrasubspecific”
Subsequent molecular studies showed that populations of L. setivimensis of the Setif Basin in Algeria and those of the Sebou Basin in Morocco were not monophyletic. Thus, mitochondrial genes of
species of Luciobarbus showed that L. setivimensis from the Setif Basin is a sister group of the Iberian species, while populations of L. setivimensis from the Sebou Basin were closer to North African species (Machordom & Doadrio, 2001b). For this reason, and in the absence of another valid name for the populations of the Sebou Basin, molecular studies have
referred to Sebou populations as Luciobarbus labiosa (Machordom & Doadrio, 1993, 2001b; Doadrio, 1994; Geiger et al., 2014).
Hence, populations from the Sebou Basin do not have a valid specific name at the present time, and we describe here this population
as a new species.
DESCRIPTION OF LUCIOBARBUS POPULATIONSTOP
The high degree of morphological and genetic differentiation of Luciobarbus populations endemic to the region of the Strait of Gibraltar in North Africa justifies the consideration of these populations
as distinct species. No available name for these populations exists, and therefore three new species are described in this
study.
Luciobarbus maghrebensis Doadrio, Perea & Yahyaoui, sp. nov.
urn:lsid:zoobank.org:act:3FE3F718-F050-44F5-93DC-C5ABFE3C8674
TYPE MATERIAL: Holotype: Fig. 6, Table 7. MNCN 279.718 female, 135.6 mm (SL); Ifrane River, Sebou Basin, Ouad Ifrane, Atlantic slope in Morocco (33.296957, -5.492639)
(Fig. 1); 27/3/2013. Collected by (Coll.) Doadrio, I.; Garzón, P.; Yahyaoui, A; González, E. G.; Perea, S.
Paratypes: Table 7. MNCN 279.711-717, 279.719-29, 279.731, 279.733-744: Thirty-one specimens from the Ifrane River, Sebou Basin, Ouad Ifrane,
Atlantic slope in Morocco (33.296957,-5.492639); 27/3/2013. Coll. Doadrio, I; Yahyaoui, A.; Garzón, P.; González, E. G.; Perea,
S. MNCN 71675-697: Twenty-three specimens from Tizguit River, Sebou Basin, Ifrane, Atlantic slope in Morocco (33.549241,-5.097144).
19/4/1991. Doadrio, I; Garzón, P.
Holotype and a series of paratypes (55 specimens) have been deposited at the Fish Collection of the Museo Nacional de Ciencias
Naturales, (Madrid, Spain).
SUPPLEMENTARY MATERIAL: Lectotype of Barbus setivimensis var. labiosa Pellegrin, 1920: MNHN-IC-1920-0215, Fez, Morocco. Coll. M. CH. Allaud. Paralectotypes of Barbus setivimensis var. labiosa Pellegrin, 1920: MNHN-IC-1920-0212 and MNHN-IC-1920-0214, Fez, Morocco.
DIAGNOSIS: Differs from other known species of Luciobarbus by the following combination of characters: 41-47 scales on the lateral line (x=43.7 Median=44); 7.5-8.5 (x=7.5 Median=7.5)
above lateral line and 4.5-6.5 (x=5.47, Median=5.5) below lateral line. Insertion of the ventral fin is posterior to dorsal
fin origin. The last single fin ray is strongly ossified and densely denticulated along its length (Append. 2-1). Postorbital
length is longer than that of other studied populations. The PrOL/PsOL ratio ranged from 0.69 to 0.84 (x=0.77). In adult specimens,
the lower lip is thick with a retracted medial lobe revealing the dentary. The ethmoid bone is narrower than its length. Vertebrae
41-43 (x=42.1, n=12), Gill Rakers (GR) 13-15 (x=14.1 Median=14). Differences in diagnostic characters among analyzed Luciobarbus populations are presented in Table 10.
DESCRIPTION: D IV 8, A III 5, P I 15-16, V I 8, C 18; LL 41-47 (x=43.7, Median=44), RSA 7.5-8.5 (x=7.5, Median=7.5), RSB 4.5-6.5
(x=5.4, Median=5.5). Pharyngeal teeth in adults 4.3.2/4.3.2., GR 13-15 (x=14.1 X=14), VE 41-43 (x=42.1, n=12). A medium-sized
species, rarely reaching 500 mm. The body is elongated relative to maximum body depth and compared to other Luciobarbus species. The head is large with respect to the body with head length 22-28% of SL. Infraorbital bones are narrow. The first
barbel did not reach the anterior edge of the eye, but, in some specimens, barbels reached the rim of the eye and extended
to half the width of the eye. The second barbel usually extended beyond the posterior rim of the eye, rarely reaching the
preopercle. The anterior barbel is 19.3-33.3%, and the second 21.2-41%, of HL. The lips are thick with the inferior usually
retracted in adults revealing the dentary bone. In some specimens, lips are not retracted and exhibited a well-developed medial
lobe. The lacrimal bone has a medium-sized manubrium. The snout is prominent, marked in some specimens, with preorbital length
7.8-10.3% of SL; postorbital length ranged from 9-13.6% SL. The iris, as in other Moroccan species of Luciobarbus is yellow pigmented at the superior border but is less conspicuous than in other species. The jugal space closes at the same
plane of the vertical of the eye, and 11-12 pores were present in the inferior branch of the pre-operculum. The dorsal fin
is posterior on the body with a predorsal distance of 48.2-55.8% SL. The profile of the dorsal fin is straight or slightly
concave, with the last single ray ossified and profusely denticulated (Fig. 7). The caudal peduncle is slightly more elongated than that in Rifian populations, with a height of 30-39.3% SL. The height
of the caudal peduncle is 1.4 to 1.98 times the length of the anal peduncle. The pectoral and ventral fins are longer in males,
and the anal fin is longer in the females. Males exhibit nuptial tubercles in the preorbital space. Ventral fins are inserted
forward to dorsal fin insertion. The caudal fin is 16.6-27.3% SL. Morphometric and meristic measurements for the holotype
and paratypes of Luciobarbus maghrebensis are represented in Table 7. The coloration of L. maghrebensis is silver-yellowish with darker fins (Fig. 6). Some specimens exhibit a darker longitudinal band in the center of the body. Juveniles present blotches, as in other Luciobarbus species. The skull is wide with a narrow ethmoid bone and a large opercle; the pharyngeal bone is wide with a long inferior
process. The lacrimal bone is well developed, and infraorbital bones are narrow. The dentary has a long anterior process,
and the maxilla has a small palatine process. The basioccipital plate is wide and triangular.
ETYMOLOGY: The species name “maghrebensis” has been selected because it is mainly distributed in the northwestern area of the Maghreb region.
DISTRIBUTION: This species is endemic to north-central Morocco, inhabiting Sebou Basin and rivers flowing into the Moulay
Bouselham Lagoon on the Atlantic slope (Fig. 1).
COMMON NAME: We propose using the English common name “Maghreb barbel” for this new species.
HABITAT AND BIOLOGY: The species can inhabit rivers of varying typology within its distribution range. Upstream it is substituted
for the trout, and in the lower courses of rivers and calm currents it is locally highly abundant. From April to May the species
migrates upstream to headwaters for spawning. The species is also present in reservoirs.
CONSERVATION: Luciobarbus maghrebensis spawning migration has been affected by dams, which present physical barriers to upstream migration. The presences of
exotic species in reservoirs, as well as poor water quality in the lower courses, due to fertilizers and pesticides, have
probably been primary causes of the decline in its population in recent years. No quantification of the decline in numbers
is available, but the species is still abundant and thriving locally. We suggest that this species should be included in the
IUCN category of Least Concern (LC).
GENETICS: Uncorrected genetic distances of mitochondrial gene cytb between Luciobarbus maghrebensis and the other analyzed species are represented in Table 6. L. maghrebensis shows 12 diagnostic positions in the cytb gene. A previous allozyme study found three diagnostic loci between L maghrebensis (GP1-2*, GP15* and PGDH-1*) and Grou population (Machordom et al., 1998).
|
 Fig. 6.— Holotype of Luciobarbus maghrebensis from the Ifrane River, Sebou Basin, Ouad Ifrane, Morocco. MNCN 279.718. Fig. 6.— Holotype of Luciobarbus maghrebensis from the Ifrane River, Sebou Basin, Ouad Ifrane, Morocco. MNCN 279.718.
Fig. 6.— Holotipo de Luciobarbus maghrebensis del río Ifrane, Cuenca del Sebou, en Ouad Ifrane, Marruecos. MNCN 279.718.
|
|
Table 7.— Morphometric and meristic measurements of the holotype and paratypes of Luciobarbus maghrebensis.
Tabla 7.— Medidas morfométricas y merísticas del holotipo y paratipos de Luciobarbus maghrebensis.
Morphometric
variables |
Holotype MNCN 279718 |
Paratypes n=54 |
| Measurements (mm) |
Range |
Mean |
Standard Deviation |
| SL |
135.6 |
70-170.6 |
109.9 |
25.2 |
| PrDD |
68.8 |
33.4-85.7 |
56.8 |
12.2 |
| PrPD |
36.2 |
18.6-43.6 |
28.2 |
6.4 |
| PrVD |
75.6 |
40.2-93.2 |
59.7 |
14.3 |
| PrAD |
104.9 |
54.7-130.7 |
84.1 |
22.3 |
| PVL |
39.9 |
19.2-52 |
32.6 |
8.4 |
| CPL |
51.3 |
24.2-67.6 |
41.4 |
10.6 |
| APL |
25.2 |
12.1-32.9 |
20.5 |
5.0 |
| BD |
34.7 |
18.7-43.6 |
28.0 |
6.4 |
| BLD |
16.1 |
7.7-18.5 |
12.3 |
2.6 |
| HL |
33.7 |
17.1-38.8 |
26.6 |
5.3 |
| PrOL |
12.2 |
6.0-14.4.1 |
9.4 |
2.1 |
| ED |
7.7 |
4.2-8.2 |
5.7 |
1.1 |
| PsOL |
14.4 |
6.8-19.1 |
13.7 |
2.4 |
| B1L |
10.0 |
3.6-11.2 |
7.0 |
1.8 |
| B2L |
14.4 |
4.6-15.6 |
9.2 |
2.6 |
| PFL |
24.4 |
10.7-30.9 |
20.2 |
4.3 |
| VFL |
21.5 |
11.5-26 |
17.6 |
3.5 |
| DFL |
17.8 |
9.1-3.1 |
15.0 |
3.1 |
| DFH |
24.8 |
11.2-30.6 |
18.6 |
4.8 |
| AFL |
8.7 |
5.1-26.8 |
8.6 |
3.0 |
| AFH |
24.4 |
10.9-31 |
18.6 |
4.2 |
| CFL |
32.6 |
17.3-40.1 |
25.7 |
5.3 |
| LL |
44 |
41-47 |
43.7 |
1.2 |
| RSA |
7.5 |
7.5-8.5 |
7.6 |
0.2 |
| RSB |
5.5 |
4.5-6.5 |
5.5 |
0.4 |
|
 Fig. 7.— Denticulated last single ray of the dorsal fin in specimen of Luciobarbus maghrebensis from the Ifrane River. Fig. 7.— Denticulated last single ray of the dorsal fin in specimen of Luciobarbus maghrebensis from the Ifrane River.
Fig. 7.— Aleta dorsal con el ultimo radio sencillo denticulado en un individuo de Luciobarbus maghrebensis del río Ifrane.
|
|
Luciobarbus rifensis Doadrio, Casal-Lopez & Yahyaoui, sp. nov.
urn:lsid:zoobank.org:act:F32CCBDD-7C77-4584-889E-1DAA0733AF43
TYPE MATERIAL: Holotype: Fig. 8, Table 8. MNCN 290.642; female, 177.2 mm (SL); Laou River, Laou Basin, Derdara, Chefchauen Province, Mediterranean slope in Morocco;
(35.118986-5.288900) (Fig. 1); 6/6/2013. Coll: Doadrio, I; Yahyaoui, A; Casal-Lopez, M; Perea, S.
Paratypes: Table 8. MNCN 290.639-641, 290.643-652, 290.655, 290.657-663, 290.665-667: Twenty-four specimens from Laou River, Laou Basin, Derdara,
Chefchaouen Province, Mediterranean slope in Morocco; (35.118986, -5.288900); 6/6/2013; Coll: Doadrio, I; Yahyaoui, A; Casal-Lopez,
M.; Perea, S. MNCN 284.939-940, 284.942-945, 284.947-948, 284.950-951, 284.953-964: Twenty-two specimens from Laou River,
Laou Basin, Beni Ferten, Tétouan Province, Mediterranean slope in Morocco (35.353254, -5.184840); 8/4/2007. Coll: Doadrio,
I; Doadrio I jr; Perea, S. MNCN 280.162-163, 280.165, 280.168, 280.170, 280.172-174, 280.176-181, 280.183, 280.185-186; 290.671-696:
Forty-three specimens from Loukos River, Loukos Basin, Souk Had, Laghdir, Chefchaouen Province, Atlantic slope in Morocco
(35.02624, -5.404660) 30/10/2011. Coll: Doadrio, I; Yahyaoui, A; Casal-Lopez, M; Garzon, P. MNCN 290.707-714, 290.716-722,
290.725-731. Twenty-two specimens from Hachef River, Hachef Basin, Dar Chaoui, Tétouan Province, Atlantic slope in Morocco
(35.526763, -5.713771); 6/6/2013. Coll: Doadrio, I; Yahyaoui, A; Casal-Lopez, M; Perea, S.
Holotype and a series of paratypes (112 specimens) have been deposited in the Fish Collection of the Museo Nacional de Ciencias
Naturales, Madrid, Spain.
DIAGNOSIS: Differs from other known species of Luciobarbus by the following combination of characters: 42-46 scales on the lateral line (x=43.9, Median=44), 8.5-9.5 scales above lateral
line (x=8.8, Median=8.5), and 3.5-5.5 scales below lateral line (x=4.7, Median=4.5). Insertions of dorsal fin and ventral fin
were situated similarly on the body or the insertion of the dorsal fin is slightly anterior to the ventral fin origin. Last
single fin ray is ossified and denticulated along its length. The denticulations at mid-height of the ray are shorter than
the ray width. In adult specimens, the lower lip is well developed with retracted medial lobe revealing the dentary. The ethmoid
bone is wider than long. Vertebrae 41-42, (x=42, n=12); gill rakers 10-15 (x=13.6 Median=13). Diagnostic characters of the studied
Luciobarbus populations are presented in Table 10.
DESCRIPTION: D IV 8, A III 5, P I 15-16, V I 8, C 18. LL 42-46 (x=43.9, Median=44); RSA 8.5-9.5 (x=8.8, Median=8.5); RSB 3.5-5.5
(x=4.7, Median=4.5). Pharyngeal teeth in adults 4.3.2/4.3.2. GR 10-15 (x=13.6 Median=13); VE 41-42 (x=42 n=12). A medium-sized
species that rarely reaches 500 mm. The body is short and deep in comparison with other Luciobarbus species with maximum body depth 22-28% SL. The head is large with respect to the body with head length 23.2-27.8% SL. Circumorbital
bones are narrow. The barbels were long with the first barbel not reaching the anterior edge of the eye. The second barbel
usually extended to the posterior edge of the eye, but did not reach the preopercle. In females, the first barbel is 14.5-36%
HL and the second 20-40.3% HL. The lips are thick with the inferior usually retracted in adults making the dentary bone visible.
Some specimens do not exhibit inferior lip retraction and show well developed lips (Fig. 9). The lacrimal bone has a short manubrium. The eye is placed anteriorly in the head; preorbital length is 7.1-10.6% SL, and
postorbital length is 10.2-12.5% SL. The snout is characteristically rounded. The iris, as in other Moroccan species of Luciobarbus, is yellow pigmented at the superior border but is less conspicuous than in other species. The jugal spacious closes at the
same vertical plane of the eye and from 11 to 12 pores were present in the inferior branch of the preopercular. The dorsal
fin was located posterior on the body, with the predorsal distance being 51.2-55.9% SL. The profile of the dorsal fin was
slightly concave, and presents the last single ray strongly ossified and denticulated. The caudal peduncle (CPL) is high and
short with a height of 32.6-39% SL. The height of the caudal peduncle is 1.2 to 2 times the length of the anal peduncle. The
pectoral and ventral fins are longer in males, and the anal fin is longer in females. Males have nuptial tubercles in the
preorbital space, but not as well developed as in other Luciobarbus species. Ventral fins are inserted in the same vertical plane as the origin of the dorsal fin or slightly posterior to the
dorsal fin. The length of the caudal fin is 14.9-26.5% SL. Morphometric and meristic measurements for the holotype and the
paratypes of the newly described species are represented in Table 8. The coloration of L. rifensis is brown-yellowish with darker fins, with the body becoming progressively paler in the ventral region (Fig. 8). Juveniles present black blotches as in other Luciobarbus species. The skull is long and narrow with a wide ethmoid bone. Infraorbitals are narrow with a large lacrimal bone. The
basioccipital plate is triangular. The palatine process of maxilla is small. The superior branch of the pharyngeal bone forms
a closed angle with respect to inferior branch.
ETYMOLOGY: The species name “rifensis” has been selected because the distribution range comprises the Rifian Mountains of Morocco.
DISTRIBUTION: The new species is endemic to north Morocco, inhabiting waters from the Loukos Basin on the western Atlantic
slope to the Laou Basin on the eastern Mediterranean slope (Fig. 1).
COMMON NAME: We propose the English common name “Rifian barbel” for this newly identified species.
HABITAT AND BIOLOGY: The species is ubiquitous and inhabits streams and rivers with substrata ranging from sandy to stony,
being absent only in small streams near the sources of rivers and in shallow waters. The species is present in reservoirs.
The spawning period is variable, but usually takes place in April and May. The species migrates upstream to spawn in cold
and oxygenated waters where the females excavate a nest in the gravel.
CONSERVATION: Luciobarbus rifensis is a common species. However, its distribution area has been extensively transformed in recent years due to construction
of dams, which also hamper upstream migration during the spawning period. In addition, reservoirs can harbor exotic species,
some piscivorous, which could be a potential threat to barbels. The area where the species occurs has and increasing interest
for tourism activities, which increases water abstraction for recreational use, leading to decreased water levels in summer
as well as water oxygen depletion. The species is currently common and locally abundant. For this reason, we suggest that
it should be included in the IUCN category as Least Concern (LC).
GENETICS: Uncorrected genetic distance based on mitochondrial gene cytb between Luciobarbus rifensis populations and the other analyzed populations are represented in Table 6. L. rifensis shows 6 diagnostic positions in the cytb gene. An earlier allozyme study found four diagnostic loci to L. rifensis (MPI, GPI-5, IDHP-2 and CK) with respect to Sebou populations (Machodom et al., 1998).
|
 Fig. 8.— Holotype of Luciobarbus rifensis from Laou river, Laou Basin, in Derdara, Morocco. MNCN 290.641. Fig. 8.— Holotype of Luciobarbus rifensis from Laou river, Laou Basin, in Derdara, Morocco. MNCN 290.641.
Fig. 8.— Holotipo de Luciobarbus rifensis del río Laou, Cuenca del Laou, en Derdara, Marruecos. MNCN 290.641.
|
|
Table 8.— Morphometric and meristic measurements of holotype and paratype series of Luciobarbus rifensis.
Tabla 8.— Medidas morfométricas y merísticas del Holotipo y Paratipos de Luciobarbus rifensis.
Morphometric
variables |
Holotype MNCN 290642 |
Paratypes n=111 |
| Measurement (mm) |
Range |
Mean |
Standard Deviation |
| SL |
177.2 |
77.2-226 |
127.2 |
41.6 |
| PrDD |
90.4 |
41.4-122.8 |
69.4 |
22.5 |
| PrPD |
45.3 |
20.9-63.3 |
34.1 |
11.4 |
| PrVD |
94.6 |
43.4-126.2 |
71.2 |
23.1 |
| PrAD |
135.3 |
58.4-173.4 |
98.4 |
32.1 |
| PVL |
50.7 |
21.9-65.4 |
37.5 |
12.0 |
| CPL |
68.6 |
26.9-86.0 |
46.7 |
15.9 |
| APL |
33.2 |
13.2-43.5 |
23.6 |
8.3 |
| BD |
45.4 |
18.4-58.2 |
32.5 |
10.6 |
| BLD |
19.1 |
8.4-21.8 |
13.6 |
3.7 |
| HL |
41.1 |
19.3-56.7 |
31.9 |
10.1 |
| PrOL |
15.7 |
6-20.3 |
11.2 |
3.5 |
| ED |
7.0 |
3.7-11.4 |
6.6 |
1.9 |
| PsOL |
18.6 |
8-27.1 |
14.4 |
5.0 |
| B1L |
8.1 |
3.0-16.3 |
7.6 |
3.3 |
| B2L |
13.3 |
4.1-22 |
10.0 |
4.2 |
| PFL |
28.4 |
12.3-42.2 |
22.3 |
7.5 |
| VFL |
23.9 |
11.1-32.3 |
18.5 |
5.5 |
| DFL |
23.2 |
9.5-28.7 |
16.1 |
4.8 |
| DFH |
31.9 |
11.2-41.9 |
21.7 |
7.1 |
| AFL |
12.5 |
4.8-17.3 |
9.4 |
3.1 |
| AFH |
31.1 |
11.7-42.1 |
20.8 |
7.7 |
| CFL |
36.7 |
16.2-49.2 |
26.9 |
8.3 |
| LL |
44 |
42-46 |
43.8 |
0.8 |
| RSA |
9.5 |
8.5-9.5 |
8.7 |
0.4 |
| RSB |
5.5 |
3.5-5.5 |
4.7 |
0.4 |
|
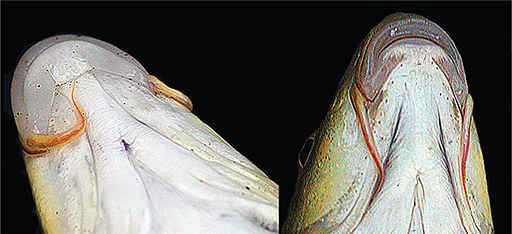 Fig. 9.— Well developed lips with medial lobe in a specimen of Luciobarbus rifensis from the Loukos River on the left and lips retracted revealing dentary in one specimen of Luciobarbus rifensis from Laou River on the right. Fig. 9.— Well developed lips with medial lobe in a specimen of Luciobarbus rifensis from the Loukos River on the left and lips retracted revealing dentary in one specimen of Luciobarbus rifensis from Laou River on the right.
Fig. 9.— A la izquierda un individuo de Luciobarbus rifensis del río Loukos con labios bien desarrollados y lóbulo medial. A la derecha un individuo de Luciobarbus rifensis del río Laou con los labios retraídos dejando ver el dentario.
|
|
Luciobarbus rabatensis Doadrio, Perea & Yahyaoui, sp. nov.
urn:lsid:zoobank.org:act:45CC4380-B532-4AEC-9050-692042FF0365
TYPE MATERIAL: Holotype: Fig. 10, Table 9. MNCN 71920, male, 129.9 mm (SL); Grou River, Bou Regreg Basin, Sebt Ait Rahhou, Atlantic slope of Morocco (33.164678, -6.366963)
(Fig. 1). Coll: P. Garzón, I. Doadrio, and A. Yahyaoui. 17/04/1991.
Paratypes: MNCN 71725-746, 71918-919, 71921-928; Thirty-two specimens from the Grou River, Bou Regreg Basin, Sebt Ait Rahhou
(33.164678, -6.366963) Morocco. Coll: P. Garzón, I. Doadrio and A. Yahyaoui. 17/04/1991
Holotype and the series of paratypes (33 specimens) have been deposited in the Fish Collection of the Museo Nacional de Ciencias
Naturales (Madrid, Spain).
DIAGNOSIS: Differs from other known species of Luciobarbus by the following combination of characters: Number of scales on LL 45-50 (x=46.5, Median=46); number of scales above lateral
line 8.5-10.5 (x=9.6, Median=9.6). Number of scales below lateral line 6.6-7.5 (x=6.7, Median=6.5). Insertion of dorsal and ventral fins is in the same vertical plane. Last single fin ray is ossified, with deep denticulations along its length. Denticulations
at mid-height of the ray are equal to or longer than ray width. In adult specimens, the inferior lip is retracted revealing
the dentary. The ethmoid bone is wider than its length. The maxilla showed a large palatine process. Vertebrae 41-42 (x=42,
n=6); gill rakers 14-12 (x=12.9). Diagnostic characters of the analyzed Luciobarbus populations are presented in Table 10.
DESCRIPTION: D IV 8, A III 5, P I 15-16, V I 8, C 18; LL 46-50 (x=46.5, Median=46), RSA 8.5-10.5 (x=9.6, Median=9.6), RSB 6.5-7.5
(x=6.7, Median=6.5). Pharyngeal teeth in adults 4.3.2/4.3.2. GR 12-14 (x=12.9 Median=13). VE 41-42 (x=42 n=6). It is a medium-sized
species that rarely reaches 500 mm. The body is slightly more elongated in comparison with L. rifensis and L. maghrebensis with maximum body depth ranging from 24.4-28.5% SL. The head is large relative to the body, similar to other Luciobarbus species, with head length 23.5-26.8% SL. The preorbital distance is short and the proportion with respect to head length
is 29.2-40.5% SL. The circumorbitals bones are narrow. The barbels are similar in size to those of L. maghrebensis and L. rifensis, but, due to a shorter snout, the first barbel usually reaches the anterior edge of the eye. The second barbel usually extends
to the posterior edge of the eye, but does not reach the preopercle. The anterior barbel is 22.1-26.3% HL and the second 22.3-32.8%
HL. The lips of adult individuals are usually retracted, and the dentary bone is visible. The lacrimal bone has a short and
high manubrium. As in other Moroccan Luciobarbus species, the iris is yellow pigmented at the superior border. The dorsal fin is placed posteriorly on the body with a predorsal
lenght of 50.9-56.3% SL. The profile of the dorsal fin is concave with the last single ray ossified with pronounced denticulation.
The caudal peduncle (CPL) is slightly more elongated than in L. maghrebensis and L. rifensis, with a height from 34.7 to 38% SL. The height of the caudal peduncle is 1.4 to 1.7 times the anal peduncle length. The pectoral
and ventral fins are longer in males, and the anal fin is longer in females. Males present nuptial tubercles in the preorbital
space. The caudal fin is long, with a lenght of 20.4-24.2% SL. Morphometric and meristic measurements for the holotype and
the paratypes of the newly described species are presented in Table 9. The colour is slightly yellow, brownish in the dorsal region and more silver in the ventral area. The skull is long and
narrow with the ethmoid bone wider than its length. The lacrimal bone is short. The basioccipital bone has a wide pentagonal
plate. The palatine process of the maxilla is more conspicuous and the pharyngeal bone is narrower than in specimens of the
other populations studied.
ETYMOLOGY: The species name “rabatensis” has been selected because its distribution area mainly comprises the Bou Regreg Basin, which flows through Rabat City.
DISTRIBUTION: This new species is endemic to north Morocco, inhabiting the Bou Regreg Basin (Fig. 1).
COMMON NAME: We propose the English common name “Rabat barbel” for this species.
HABITAT AND BIOLOGY: As other barbels of Morocco, with the exception of L. magniatlantis, L. rabatensis inhabits rivers of differing typologies. The species is present in reservoirs. Spawning takes places at the end of April
and the beginning of May. At that time, individuals migrate upstream for spawning in cold and oxygenated waters where the
females excavate a nest in the gravel.
CONSERVATION: The habitat of Luciobarbus rabatensis has been extensively transformed with the growth of Rabat City, urban development, water usage, increased pollution linked
to agriculture, construction of dams and reservoirs, and introduction of exotic species. Although, an abundant population
of this species is still found, it is suffering a slight decline. For this reason, we suggest that this species should be
included in the IUCN category of Near Threatened (NT).
GENETICS: Uncorrected genetic distances between Luciobarbus rabatensis and the sister population of Luciobarbus ksibi to mitochondrial gene cytb was 1.7%. Luciobarbus rabatensis shows 6 diagnostic positions in the cytb gene. A previous allozyme study found one diagnostic locus (GPI-2* 118) with respect to other African Luciobarbus.
|
 Fig. 10.— Holotype of Luciobarbus rabatensis from Grou River, Bou Regreg Basin, Sebt Ait Rahhou, (Morocco). MNCN 71920. Fig. 10.— Holotype of Luciobarbus rabatensis from Grou River, Bou Regreg Basin, Sebt Ait Rahhou, (Morocco). MNCN 71920.
Fig. 10.— Holotipo de Luciobarbus rabatensis del río Grou, cuenca del Bou Regreg en Sebt Ait Rahhou, Marruecos. MNCN 71920.
|
|
Table 9.— Morphometric and meristic measurements of holotype and paratypes of Luciobarbus rabatensis.
Tabla 9.— Medidas morfometicas y merísticas del Holotipo y Paratipos de Luciobarbus rabatensis.
| Morphometric variables |
Holotype MNCN 71920 |
Paratypes n=32 |
| Measurement in mm |
Range |
Mean |
Standard Deviation |
| SL |
129.9 |
53.1-113.6 |
74.8 |
14.5 |
| PrDD |
66.1 |
29.1-59.9 |
40.4 |
7.3 |
| PrPD |
33.8 |
14.4-30.4 |
19.5 |
4.3 |
| PrVD |
68.2 |
28.8-60.7 |
39.8 |
8 |
| PrAD |
96.5 |
39.1-84.5 |
55.5 |
10.8 |
| PVL |
34.7 |
14.3-30.8 |
20.8 |
4 |
| CPL |
49.5 |
18.9-40.5 |
26.8 |
5.4 |
| APL |
24.7 |
9.1-21.7 |
13.8 |
2.8 |
| BD |
31.7 |
14.9-28.8 |
16.7 |
3.5 |
| BLD |
15.2 |
6.3-14.1 |
8.9 |
1.8 |
| HL |
32.1 |
14.2-27.3 |
18.8 |
3.4 |
| PrOL |
12.8 |
4.1-11-1 |
6.3 |
1.6 |
| ED |
6.3 |
3.5-5.6 |
4.4 |
0.6 |
| PsOL |
16.3 |
6.6-13.7 |
9.2 |
1.7 |
| B1L |
8.2 |
3.1-6.7 |
4.6 |
0.8 |
| B2L |
9.9 |
4.1-8.4 |
5.7 |
1.1 |
| PFL |
25.8 |
11.4-22.5 |
15.4 |
2.7 |
| VFL |
21.6 |
9.2-18.6 |
12.7 |
2.2 |
| DFL |
16.5 |
7.4-15 |
10.1 |
1.9 |
| DFH |
24.5 |
10.3-21.9 |
14.3 |
2.8 |
| AFL |
9.3 |
3.7-8.1 |
5.2 |
1.1 |
| AFH |
24.3 |
10-21.1 |
13.9 |
2.5 |
| CFL |
26.5 |
12.7-24 |
17.1 |
2.7 |
| LL |
46 |
45-50 |
46.5 |
1.3 |
| RSA |
9.5 |
8.5-10.5 |
9.6 |
0.4 |
| RSB |
6.5 |
6.5-7.5 |
6.7 |
0.4 |
Table 10.— Diagnostic morphological characters for the four species studied.
Tabla 10.— Caracteres morfológicos diagnósticos
para las cuatro especies estudiadas.
|
Last Dorsal Fin Ray |
Scales |
Origin dorsal fin |
Postorbital length |
Ethmoid bone |
Pharyngeal bone |
Infraorbitals |
| L. rifensis |
Highly ossified and densely denticulate
Denticulations are pesent most of the ray length
Denticulations are shorter than the width of the ray |
LL≤46
RSA≤8.5
RSB≤5.5 |
Vertical to the origin of pelvic fin |
Short |
Wider than long |
Upper branch hooked
Lower branch wide |
Lachrymal large
Infraorbital narrow
Second infraorbital large |
| L. maghrebensis |
Highly ossified and densely denticulate
Denticulations are pesent along the entire length of the ray
Denticulations are shorter than the width of the ray |
LL≤46
RSA≤7.5
RSB≤5.5 |
Anterior to the origin of pelvic fin |
Large |
Longer than wide |
Upper branch not hooked
Lower branch wide |
Lachrymal large
Infraorbitals narrow
Second infraorbital short |
| L. rabatensis |
Ossified with deep well-separated denticulations
Denticulations length are equal to or greater than the width of the ray |
LL≥46
RSA≥5.5
RSB≥6.5 |
Vertical to the origin of ventral fin |
Short |
Wider than long
Lower branch thin |
Upper branch not hooked
Infraorbitals narrow |
Lachrymal short
Second infraorbital short |
| L. ksibi |
Ossified and feebly sawn
Denticulations are absent from the upper third of the ray |
LL≤46
RSA≤8.5
RSB≤5.5 |
Vertical to the origin of ventral fin |
Short |
Wider than long |
Upper branch hooked
Lower branch wide
Pharyngeal bone robust |
Lachrymal short
Infraorbitals wide
Second infraorbital short |
AcknowledgmentsTOP
Many persons have participated in the field sampling trips. We warmly thank J. Cubo, M. Merino, J. L. González, P. Garzón,
I. Doadrio Jr., A. Doadrio, A. Perdices, Y. Bernat, and S. El Gharbi. We would also like to thank L. Alcaraz, involved in
lab work, the curator of the ichthyological collection of National Museum of the Natural Sciences (MNCN-CSIC), G. Solís and
also C. Parejo for her technical assistance at the lab of non-destructive techniques with the computerized Tomography scan
(CTscan) at the MNCN-CSIC. This project was funded by MESRSFC and the CNRST from Morocco to the Project N°PPR/2015/2 “Impact
des changements climatiques sur la diversité génétique des poissons des eaux douces du Maroc”. Permission for fish collection
was provided by the High Commissioner for Water, Forest and the Fight Against Dessertification (HCEFLCD) of Morocco.
ReferencesTOP
| ○ |
Akaike, H., 1973. Information theory and an extension of the Maximum Likelihood principle. In: B. N. Petrov & F. Csaki (eds.).
Proceedings of the second International Symposium on Information Theory. Akademini Kiado. Budapest: 267-281.
|
| ○ |
Almaça, C., 1966. Sur la systématique des Barbeaux marocaines (Pisces, Cyprinidae, Barbus). Arquivos do Museo Bocage, 7: 111-114.
|
| ○ |
Almaça, C., 1968. Revision critique de quelques types de Cyprinidés d’Europe et d’Afrique du Nord des collections du Muséum
National d’Histoire Naturelle. Bulletin du Muséum National d’Histoire Naturelle, 40: 1116-1144.
|
| ○ |
Almaça, C., 1970. Sur les barbeaux (genre et sous-genre Barbus) de l’Afrique du Nord. Bulletin du Muséum National d’Histoire Naturelle, 42: 141-158.
|
| ○ |
Berrebi, P., Kraiem, M. M., Doadrio, I., El Gharbi, S. & Cattaneo-Berrebi, G., 1995. Ecological and genetic differentiation
of Barbus callensis populations in Tunisia. Journal of Fish Biology, 47(5): 850-864. http://dx.doi.org/10.1111/j.1095-8649.1995.tb06007.x |
| ○ |
Banarescu, P. & Bogutskaya N. G., 2003. The Freshwater fishes of Europe. Vol 5/II. Cyprinidae II. Part II: Barbus. Aula Verlag. Wiebelsheim. 454 pp.
|
| ○ |
Burnaby, T. P., 1966. Growth-invariant discriminant functions and generalized distances. Biometrics, 22: 96-110. http://dx.doi.org/10.2307/2528217 |
| ○ |
Doadrio, I., 1990. Phylogenetics relationships and classification of western paleartic species of the genus Barbus (Osteichthyes, Cyprinidae). Aquatic Living Resources, 3: 265-282.
|
| ○ |
Doadrio, I., 1994. Freshwater fish fauna of North Africa and its biogeography. Annals of the Royal Central African Museum (Zoology), 275: 21-34.
|
| ○ |
Doadrio, I., Bouhadad, R. & Machordom, A., 1998. Genetic differentiation and biogeography in Saharan populations of the genus
Barbus (Osteichthyes, Cyprinidae). Folia Zoologica, 47: 7-20.
|
| ○ |
Doadrio, I. & Carmona, J. A., 2003. Testing freshwater Lago Mare Dispersal Theory on the Phylogeny Relationships of Iberian
Cyprinid Genera Chondrostoma and Squalius (Cyprinoformes, Cyprinidae). Graellsia, 59(2-3): 457-473. http://dx.doi.org/10.3989/graellsia.2003.v59.i2-3.260 |
| ○ |
Doadrio, I., & Carmona, J. A., 2006. Phylogenetic overview of the genus Squalius (Actinopterygii, Cyprinidae) in the Iberian Peninsula, with description of two new species. Cybium, 30(3): 199-214.
|
| ○ |
Doadrio, I., Carmona, J. A. & Machordom, A., 2002. Haplotypes diversity and phylogenetic relationships among the Iberian barbels
(Barbus, Cyprinidae) reveal two evolutionary lineages. Journal of Heredity, 93(2): 140-147. http://dx.doi.org/10.1093/jhered/93.2.140 |
| ○ |
Doadrio, I. & Elvira, B., 2007. A new species of the genus Achondrostoma Robalo, Almada, Levy & Doadrio, 2007 (Actinopterygii, Cyprinidae) from western Spain. Graellsia, 63(2): 295-304. http://dx.doi.org/10.3989/graellsia.2007.v63.i2.96 |
| ○ |
Doadrio, I., Kottelat, M. & de Sostoa, A., 2007a. Squalius laietanus, a new species of cyprinid fish from north-eastern Spain and southern France (Teleostei: Cyprinidae). Ichthyological Exploration of Frehwaters, 18(3): 247-256.
|
| ○ |
Doadrio, I. & Madeira, M. J., 2004. A new species of the genus Gobio Cuvier 1816 (Actynopterigii, Cyprinidae) from the Iberian Peninsula and south of France. Graellsia, 60(1): 107-116. http://dx.doi.org/10.3989/graellsia.2004.v60.i1.197 |
| ○ |
Doadrio, I., Perea, S. & Alonso, F., 2007b. A new species of the genus Squalius Bonaparte, 1837 (Actinipterygii, Cyprinidae) from the Tagus River Basin (Central Spain). Graellsia, 63(1): 89-100. http://dx.doi.org/10.3989/graellsia.2007.v63.i1.83 |
| ○ |
Geiger, M. F., Herder, F., Monaghan, F. T., Almada, V., Barbieri, R., Bariche, M., Berrebi, P., Bohlen, J., Casal-Lopez, M.,
Delmastro, G. B., Denys, G. P. J., Dettai, A., Doadrio, I., Kalogianni, E., Kärst, H., Kottelat, A., Kovačić, M., Laporte,
M., Lorenzoni, M., Marčić, Z., Özuluğ, M., Perdices, A., Perea, S., Persat, H., Porcelotti, S., Puzzi, C., Robalo, J., Šanda,
R., Schneider, M., Šlechtová, V., Stumboudi, M., Walter, S. & Freyhof, J., 2014. Spatial heterogeneity in the Mediterranean
biodiversity hotspot affects barcoding accuracy of its freshwater fishes. Molecular Ecology Resources, 14(6): 1210-1221. http://dx.doi.org/10.1111/1755-0998.12257 |
| ○ |
Domínguez-Domínguez, O., Pompa-Domínguez, A. & Doadrio, I., 2007. A new species of the genus Yuriria Jordan & Evermann, 1896 (Actynopterigii, Cyprinidae) from the Ameca basin of the Central Mexican Plateau. Graellsia, 63(2): 259-271. http://dx.doi.org/10.3989/graellsia.2007.v63.i2.93 |
| ○ |
Domínguez-Domínguez, O., Pérez-Rodríguez, R., Escalera-Vázquez, L. H. & Doadrio, I., 2009. Two new species of the genus Notropis Rafinesque, 1817 (Actinopterygii, Cyprinidae) from the Lerma River Basin in Central Mexico. Hidrobiológica, 19(2): 159-172.
|
| ○ |
Griffiths, H. I., Krystufec, B. & Reed, J. M., 2004. Balkan Biodiversity Pattern and Process in the European Hotspot. Kluwer Academic Publisher. Dordrecht. 361 pp.
|
| ○ |
Karaman, M. S., 1971. Süsswasserfische der Türkei. 8. Teil: Revision der Barben Europas, Vorderasiens und Nordafrikas. Mitteilungen aus dem Hamburgischen Zoologischen Museum und Institut, 67: 175-254.
|
| ○ |
Kottelat, M. & Freyhof, J., 2007. Handbook of European freshwater fishes. Cornol. Berlin. 646 pp.
|
| ○ |
Machordom, A., Bouhadad, I. & Doadrio, I., 1998. Allozyme variation and evolutionary history of North African populations
of the genus Barbus (Osteichthyes, Cyprinidae). Diversity and Distributions, 4: 217-234.
|
| ○ |
Machordom, A. & Doadrio, I., 1993. Phylogenie et taxonimie des barbeaux nord-africanes. Cahiers d´Ethologie, 13(2): 218.
|
| ○ |
Machordom, A., Doadrio, I. & Berrebi, P., 1995. Phylogeny and evolution of the genus Barbus in the Iberian Peninsula as revealed by allozyme electrophoresis. Journal of Fish Biology, 47(2): 211-236. http://dx.doi.org/10.1111/j.1095-8649.1995.tb01890.x |
| ○ |
Machordom, A. & Doadrio, I., 2001a. Evolutionary history and speciation modes in the cyprinid genus Barbus. Proceedings of the Royal Society of London. Series B: Biological Sciences, 268(1473): 1297-1306. http://dx.doi.org/10.1098/rspb.2001.1654 |
| ○ |
Machordom, A. & Doadrio, I., 2001b. Evidence of a Cenozoic-Betic-Kabilian connection based on freshwater fish phylogeography
(Luciobarbus, Cyprinidae). Molecular Phylogenetics and Evolution, 18(2): 252-263. http://dx.doi.org/10.1006/mpev.2000.0876 |
| ○ |
Pellegrin, J., 1920. Poissons du Maroc recueillis par M. Ch. Alluaud. Bulletin du Muséum National d‘Histoire Naturelle, 26(7): 612-613.
|
| ○ |
Pellegrin, J., 1922. Poissons recueillis par M. Ch. Alluaud dans la région du Sous (Maroc). Bulletin de la Société des sciences naturelles du Maroc, 2: 103-106.
|
| ○ |
Posada, D., 2008. jModelTest: Phylogenetic Model Averaging. Molecular Biology and Evolution, 25(7): 1253-1256. http://dx.doi.org/10.1093/molbev/msn083 |
| ○ |
Robalo, J. I., Almada, V. C., Sousa Santos, C., Moreira, M. I. & Doadrio, I., 2005. New species of the genus Chondrostoma Agassiz, 1832 (Actynopterigii, Cyprinidae) from western Portugal. Graellsia, 61(1): 15-28. http://dx.doi.org/10.3989/graellsia.2005.v61.i1.3 |
| ○ |
Rohlf, F. J., 2003. TpsDig, Digitize Landmarks and Outlines. Department of Ecology and Evolution, State University of New York. Stony Brook.
|
| ○ |
Rohlf, F. J. & Bookstein, F. L., 1987. A comment on shearing as a method for “size correction”. Systematic Zoology, 36(4): 356-367. http://dx.doi.org/10.2307/2413400 |
| ○ |
Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D. L., Darling, A., Höhna, S., Larget, B., Liu, L., Suchard, M. A. &
Huelsenbeck, J. P., 2012. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choise Across Large Model Space.
Systematic Biology, 61(3): 539-542. http://dx.doi.org/10.1093/sysbio/sys029 |
| ○ |
Tamura, K., Stecher, G., Peterson, D., Filipski, A. & Kumar, S., 2013. MEGA6: Molecular Evolutionary Genetics Analysis (MEGA)
Software version 6.0. Molecular Biology and Evolution, 24(8): 1596-1599. http://dx.doi.org/10.1093/molbev/mst197 |
| ○ |
Tsigenopoulos, C. S., Durand, J. D., Ünlu, E. & Berrebi, P., 2003. Rapid radiation of the Mediterranean Barbus species (Cyprinidae) after the Messinian Salinity Crisis of the Mediterranean Sea, inferred from mitochondrial phylogenetic
analysis. Biological Journal of the Linnean Society, 80(2): 207-222. http://dx.doi.org/10.1046/j.1095-8312.2003.00237.x |
| ○ |
Yang, L., Sado, T., Vincent Hirt, M., Pasco-Viel, E., Arunachalam, M., Li, J., Wang, X., Freyhof, J., Saitoh, K., Simons,
A. M., Miya, M., He, S. & Mayden, R. L., 2015. Phylogeny and polyploidy: resolving the classification of cyprinine fishes
(Teleostei: Cypriniformes). Molecular Phylogenetics and Evolution, 85: 97-116. http://dx.doi.org/10.1016/j.ympev.2015.01.014 |
| ○ |
Zardoya, R. & Doadrio, I., 1999. Molecular evidence on the evolutionary and biogeographical patterns of European cyprinids.
Journal of Molecular Evolution, 49(2): 227-237. http://dx.doi.org/10.1007/PL00006545 |
| ○ |
Zardoya, R., Economidis, P. S. & Doadrio, I., 1999. Phylogenetic relationshipes of Greek Cyprinidae: molecular evidence for
at least two origins of the Greek cyprinid fauna. Molecular Phylogenetics and Evolution, 13(1): 122-131. http://dx.doi.org/10.1006/mpev.1999.0630 |
APPENDIXTOP
Appendix 1.— Kruskal-Wallis test and Non-parametric Mann-Whitney’s pairwise comparisons for all populations. Values
of Kruskal-Wallis test (H) below variables. Values of Mann-Whitney test are below the diagonal. Median in the diagonal of
each variable. Significant differences p<0.05 (*); p<0.01 (**). Acronyms are defined in the Material and Methods.
Apéndice 1.— Test de Kruskal-Wallis y análisis no paramétrico de Mann-Whitney para todas las poblaciones. Valores para el test de Kruskal-Wallis
(H) debajo de las variables. Valores de Mann-Whitney por debajo de la diagonal. Valor de la Mediana en la diagonal de cada
variable. Diferencias significativas p<0,05 (*); p<0,01 (**). Las abreviaturas están descritas en el epígrafe de Material
y Métodos.
| Variables |
Populations |
Rif (n=112) |
Sebou (n=55) |
Bou Regreg (n=33) |
L. ksibi (n=23)
|
| SL (H=65.99**) |
Rif |
140.46 |
|
|
|
|
Sebou |
0.018* |
106.13 |
|
|
|
Bou-Regreg |
<0.0001** |
<0.0001** |
71.88 |
|
|
Kasab |
<0.0001** |
0.005** |
0.0006** |
88.97 |
| SL/PrDD (H=55.87**) |
Rif |
1.876 |
|
|
|
|
Sebou |
<0.0001** |
1.934 |
|
|
|
Bou-Regreg |
0.005* |
<0.0001** |
1.843 |
|
|
Kasab |
0.206 |
<0.0001** |
0.624 |
1.863 |
| SL/PrPD (H=20.44**) |
Rif |
3.768 |
|
|
|
|
Sebou |
<0.001** |
3.682 |
|
|
|
Bou-Regreg |
0.021* |
0.379 |
3.818 |
|
|
Kasab |
0.002** |
0.8084 |
0.34 |
3.88 |
| SL/PrVD (H=97.1**) |
Rif |
1.793 |
|
|
|
|
Sebou |
<0.0001** |
1.828 |
|
|
|
Bou-Regreg |
<0.0001** |
<0.0001** |
1.87 |
|
|
Kasab |
<0.0001** |
<0.0001** |
<0.0001* |
1.919 |
| SL/PrAD (H=86.63**) |
Rif |
1.304 |
|
|
|
|
Sebou |
0.183 |
1.311 |
|
|
|
Bou-Regreg |
<0.0001** |
<0.0001** |
1.346 |
|
|
Kasab |
<0.0001** |
<0.0001** |
0.938 |
1.347 |
| PrVD/PrDD (H=89.65**) |
Rif |
1.045 |
|
|
|
|
Sebou |
0.051 |
1.065 |
|
|
|
Bou-Regreg |
<0.0001** |
<0.0001** |
0.981 |
|
|
Kasab |
<0.0001** |
<0.0001** |
0.02* |
0.964 |
| SL/PVL (H=22.62**) |
Rif |
3.474 |
|
|
|
|
Sebou |
0.277 |
3.415 |
|
|
|
Bou-Regreg |
0.002** |
<0.0001** |
3.601 |
|
|
Kasab |
0.005** |
<0.0001** |
0.414 |
3.63 |
| SL/CPL (H=41.94**) |
Rif |
2.818 |
|
|
|
|
Sebou |
<0.0001** |
2.699 |
|
|
|
Bou-Regreg |
0.301 |
<0.0001** |
2.798 |
|
|
Kasab |
0.928 |
<0.0001** |
0.481 |
2.804 |
| SL/APL (H=19.43**) |
Rif |
5.561 |
|
|
|
|
Sebou |
0.648 |
5.587 |
|
|
|
Bou-Regreg |
0.003** |
0.06 |
5.39 |
|
|
Kasab |
<0.0001** |
0.003** |
0.093 |
5.31 |
| CPL/BLD (H=68.72**) |
Rif |
3.275 |
|
|
|
|
Sebou |
0.662 |
3.187 |
|
|
|
Bou-Regreg |
<0.0001** |
<0.0001** |
3.003 |
|
|
Kasab |
<0.0001** |
<0.0001** |
<0.0001** |
2.773 |
| APL/BLD (H=27.44) |
Rif |
1.642 |
|
|
|
|
Sebou |
0.008** |
1.576 |
|
|
|
Bou-Regreg |
<0.0001** |
0.205 |
1.546 |
|
|
Kasab |
<0.0001** |
0.006 |
0.04* |
1.495 |
| SL/BD (H=44.26) |
Rif |
3.999 |
|
|
|
|
Sebou |
0.752 |
3.991 |
|
|
|
Bou-Regreg |
<0.0001** |
<0.0001** |
3.829 |
|
|
Kasab |
<0.0001** |
<0.0001** |
0.2001 |
3.75 |
| SL/BLD (H=44.76**) |
Rif |
9.061 |
|
|
|
|
Sebou |
0.752 |
8.719 |
|
|
|
Bou-Regreg |
<0.0001** |
<0.0001** |
8.378 |
|
|
Kasab |
<0.0001** |
<0.0001** |
0.2 |
7.891 |
| SL/HL (H=10.4*) |
Rif |
3.981 |
|
|
|
|
Sebou |
0.145 |
4.044 |
|
|
|
Bou-Regreg |
0.89 |
0.107 |
3.992 |
|
|
Kasab |
<0.0001** |
<0.0001** |
<0.0001** |
3.811 |
| HL/PrOL (H=27.43) |
Rif |
2.73 |
|
|
|
|
Sebou |
0.044* |
2.788 |
|
|
|
Bou-Regreg |
<0.0001** |
0.0007** |
2.98 |
|
|
Kasab |
0.397 |
0.624 |
0.002** |
2.77 |
| HL/PsOL (H=151.5**) |
Rif |
2.205 |
|
|
|
|
Sebou |
<0.0001** |
1.606 |
|
|
|
Bou-Regreg |
<0.0001** |
<0.0001** |
2.025 |
|
|
Kasab |
0.67 |
<0.0001** |
<0.0001** |
2.187 |
| SL/PrOL (36.47**) |
Rif |
10.965 |
|
|
|
|
Sebou |
0.002** |
11.364 |
|
|
|
Bou-Regreg |
<0.0001** |
<0.0001** |
12.06 |
|
|
Kasab |
0.701 |
<0.0001** |
<0.0001** |
11.122 |
| SL/PsOL (153.8**) |
Rif |
8.867 |
|
|
|
|
Sebou |
<0.0001** |
6.491 |
|
|
|
Bou-Regreg |
<0.0001** |
<0.0001** |
8.127 |
|
|
Kasab |
0.14 |
<0.0001** |
<0.0001** |
8.622 |
| PsOL/PrOL (H=153.9) |
Rif |
1.248 |
|
|
|
|
Sebou |
<0.0001** |
1.727 |
|
|
|
Bou-Regreg |
<0.0001** |
<0.0001** |
1.462 |
|
|
Kasab |
0.745 |
<0.0001** |
<0.0001** |
1.265 |
| SL/ED (H=51.66**) |
Rif |
20.004 |
|
|
|
|
Sebou |
0.936 |
19.71 |
|
|
|
Bou-Regreg |
<0.0001** |
<0.0001** |
17.1 |
|
|
Kasab |
0.004** |
0.009** |
0.303 |
17.37 |
| HL/L1B (H=22.49**) |
Rif |
4.032 |
|
|
|
|
Sebou |
0.29 |
3.9 |
|
|
|
Bou-Regreg |
0.546 |
0.011* |
4.06 |
|
|
Kasab |
0.0005** |
0.0002** |
<0.0001** |
3.545 |
| PrO/L1B (H=16.57**) |
Rif |
1.459 |
|
|
|
|
Sebou |
0.114 |
1.419 |
|
|
|
Bou-Regreg |
0.095 |
0.365 |
1.359 |
|
|
Kasab |
0.0005** |
0.001** |
0.007** |
1.293 |
| HL/L2B (H=48.31**) |
Rif |
3.154 |
|
|
|
|
Sebou |
0.037* |
3.001 |
|
|
|
Bou-Regreg |
0.085 |
0.0001 |
3.29 |
|
|
Kasab |
<0.0001** |
<0.0001** |
<0.0001** |
2.571 |
| PsOL/L2B (H=122.3**) |
Rif |
1.434 |
|
|
|
|
Sebou |
<0.0001** |
1.872 |
|
|
|
Bou-Regreg |
0.0003** |
<0.0001** |
1.612 |
|
|
Kasab |
<0.0001** |
<0.0001** |
<0.0001** |
1.17 |
| HL/PFL (H=104.1**) |
Rif |
1.43 |
|
|
|
|
Sebou |
<0.0001** |
1.318 |
|
|
|
Bou-Regreg |
<0.0001** |
<0.0001** |
1.221 |
|
|
Kasab |
<0.0001** |
<0.0001** |
0.104 |
1.179 |
| HL/VFL (H=133.2**) |
Rif |
1.721 |
|
|
|
|
Sebou |
<0.0001** |
1.524 |
|
|
|
Bou-Regreg |
<0.0001** |
0.2194 |
1.489 |
|
|
Kasab |
<0.0001** |
<0.0001** |
<0.0001** |
1.352 |
| HL/CFL (H=78.93**) |
Rif |
1.185 |
|
|
|
|
Sebou |
<0.0001** |
1.032 |
|
|
|
Bou-Regreg |
<0.0001** |
<0.0001** |
1.09 |
|
|
Kasab |
<0.0001** |
0.933 |
0.006** |
1.028 |
| LL (H=72.21**) |
Rif |
44 |
|
|
|
|
Sebou |
0.1428 |
44 |
|
|
|
Bou-Regreg |
<0.0001** |
<0.0001** |
46 |
|
|
Kasab |
0.605 |
0.681 |
0** |
44 |
| RSA (H=145.8**) |
Rif |
8.5 |
|
|
|
|
Sebou |
<0.0001** |
7.5 |
|
|
|
Bou-Regreg |
<0.0001** |
<0.0001** |
9.5 |
|
|
Kasab |
0.0004** |
<0.0001** |
<0.0001** |
8.5 |
| RSB (H=123.7**) |
Rif |
4.5 |
|
|
|
|
Sebou |
<0.0001** |
5.5 |
|
|
|
Bou-Regreg |
<0.0001** |
<0.0001** |
6.5 |
|
|
Kasab |
0.025 |
<0.0001** |
<0.0001** |
4.5 |
Appendix 2.— Osteological features.
Apéndice 2.— Características osteológicas.TOP
|
 Appendix 2-1.— Last single dorsal fin ray in adult specimens (SL>120 mm) of the studied populations. A: Rifian population
(Laou River). B: Sebou Basin (Ifrane River). C: Bou Regreg (Grou River). D: Luciobarbus ksibi (Kasab River). Appendix 2-1.— Last single dorsal fin ray in adult specimens (SL>120 mm) of the studied populations. A: Rifian population
(Laou River). B: Sebou Basin (Ifrane River). C: Bou Regreg (Grou River). D: Luciobarbus ksibi (Kasab River).
Apéndice 2-1.— Último radio sencillo de la aleta dorsal en individuos adultos (SL>120 mm) de las diferentes
poblaciones estudiadas. A: población del Rif (río Laou). B: Cuenca del Sebou (río Ifrane). C: Cuenca del Bou Regreg (río Grou).
D: Luciobarbus ksibi (río Kasab).
|
|
|
 Appendix 2-2.— Dorsal view of the skull of the populations under study. Arrows indicate width of the ethmoid bone. A: Rifian
population (Laou River) B: Sebou Basin (Ifrane River) C: Bou Regreg (Grou River), D: Luciobarbus ksibi (Kasab River). Appendix 2-2.— Dorsal view of the skull of the populations under study. Arrows indicate width of the ethmoid bone. A: Rifian
population (Laou River) B: Sebou Basin (Ifrane River) C: Bou Regreg (Grou River), D: Luciobarbus ksibi (Kasab River).
Apéndice 2-2.— Cráneo de las diferentes poblaciones estudiadas. Entre flechas se señala la diferente anchura
del etmoides. A: población del Rif (río Laou). B: Cuenca del Sebou (río Ifrane). C: Cuenca del Bou Regreg (río Grou). D: Luciobarbus ksibi (río Kasab).
|
|
|
 Appendix 2-3.— Lateral view of the skull of the studied populations. Arrows show the length of the opercular bone. A: Rifian
population (Laou River). B: Sebou Basin (Ifrane River). C: Bou Regreg (Grou River). D: Luciobarbus ksibi (Kasab River). Appendix 2-3.— Lateral view of the skull of the studied populations. Arrows show the length of the opercular bone. A: Rifian
population (Laou River). B: Sebou Basin (Ifrane River). C: Bou Regreg (Grou River). D: Luciobarbus ksibi (Kasab River).
Apéndice 2-3.— Cráneo de las diferentes poblaciones estudiadas. Entre flechas se muestra la longitud del opercular.
A: población del Rif (río Laou). B: Cuenca del Sebou (río Ifrane). C: Cuenca del Bou Regreg (río Grou). D: Luciobarbus ksibi (río Kasab).
|
|
|
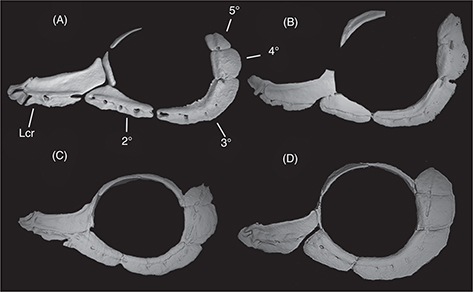 Appendix 2-4.— Infraorbital bones of the studied populations. A: Rifian population (Laou River). B: Sebou Basin (Ifrane River).
C: Bou Regreg (Grou River). D: Luciobarbus ksibi (Kasab River). Lcr = Lacrymal. 2°-5°: Infraorbitals. Appendix 2-4.— Infraorbital bones of the studied populations. A: Rifian population (Laou River). B: Sebou Basin (Ifrane River).
C: Bou Regreg (Grou River). D: Luciobarbus ksibi (Kasab River). Lcr = Lacrymal. 2°-5°: Infraorbitals.
Apéndice 2-4.— Huesos infraorbitarios de las diferentes poblaciones estudiadas.
A: población del Rif (río Laou). B: Cuenca del Sebou (río Ifrane). C: Bou Regreg (río Grou). D: Luciobarbus ksibi (río Kasab). Lcr: Lacrimal. 2°-5° Infraorbitales.
|
|
|
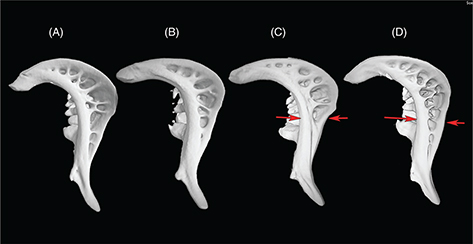 Appendix 2-5.— Pharyngeal teeth of the studied populations. The arrows show the width of the pharyngeal bone. A: Rifian population
(Laou River). B: Sebou Basin (Ifrane River). C: Bou Regreg (Grou River). D: Luciobarbus ksibi (Kasab River). Appendix 2-5.— Pharyngeal teeth of the studied populations. The arrows show the width of the pharyngeal bone. A: Rifian population
(Laou River). B: Sebou Basin (Ifrane River). C: Bou Regreg (Grou River). D: Luciobarbus ksibi (Kasab River).
Apéndice 2-5.— Dientes faríngeos de las diferentes poblaciones estudiadas. Entre flechas la anchura del hueso
faríngeo. A: población del Rif (río Laou). B: Cuenca del Sebou (río Ifrane). C: Cuenca del Bou Regreg (río Grou). D: Luciobarbus ksibi (río Kasab).
|
|
Fig. 1.— Geographic distribution of Luciobarbus spp. and sampling localities. The numbers on map correspond to localities in Table 1.