A NEW IRANIAN SPECIES OF UROLEUCON MORDVILKO, 1914 (HEMIPTERA, APHIDIDAE) FROM SPECIMENS IN THE NATURAL HISTORY MUSEUM COLLECTION
Juan M. Nieto Nafría* & Nicolás Pérez Hidalgo*,**
* Departamento de Biodiversidad y Gestión Ambiental; Universidad de León. 24071 León (Spain). e-mail 1: jmnien@unileon.es.
urn:lsid:zoobank.org:author:81F0702F-538F-4AB4-92A9-74580A562221
** e-mail 2: nperh@unileon.es. urn:lsid:zoobank.org:author:C134E648-51C1-48A2-8676-2E9517657FAE
| |
ABSTRACT
Uroleucon (Uromelan) helichrysi sp. n. (Hemiptera, Aphididae, Aphidinae: Macrosiphini) is established from Iranian apterous and alate viviparous females caught
on Helichrysum sp. (Asteraceae) and preserved in the collection of the Natural History Museum (BMNH) in London. The number of setae on the
first tarsal segments (5), the number of caudal setae (20 to 28), the presence of abdominal marginal tubercles on abdominal
segments 2 to 4, and the size of the cells of the siphuncular reticulation (relatively small) allow the new species to be
distinguished from other Palaeartic species of the subgenus Uromelan. Blackman and Eastop’s key to apterae on Helichrysum is modified to include the new species.
urn:lsid:zoobank.org:pub:E2B89D59-05FD-4A9E-AD78-B3C302219A10
Key words: Aphids;
Macrosiphini;
Iran;
Helichrysum;
Asteraceae.
|
| |
RESUMEN
Nueva especie iraní de Uroleucon Mordvilko, 1914 (Hemiptera, Aphididae) descrita a partir de ejemplares de la colección del Natural History Museum
Se establece la especie Uroleucon helichrysi n. sp. (Hemiptera: Aphididae: Aphidinae: Macrosiphini) con la descripción de sus hembras vivíparas ápteras y aladas recogidas en
Irán sobre Helichrysum sp. (Asteraceae), conservadas en la colección del Natural History Museum de Londres. La nueva especie se diferencia de otras especies paleárticas del subgénero Uromelan por la cantidad de setas en el primer segmento tarsal (5), la cantidad de setas caudales (20 a 28), por el tamaño de las celdillas de la
reticulación cornicular (que son relativamente pequeñas) y por la presencia de papilas marginales (presentes en los segmentos
abdominales 2 a 4) Se incluye una modificación a la clave de Blackman and Eastop para las ápteras que viven sobre Helichrysum.
Palabras clave: Pulgones;
Áfidos;
Macrosiphini;
Irán;
Helichrysum;
Asteraceae.
|
IntroductionTOP
It is well established that an significant part of the biodiversity is as yet undescribed, but it is not well known that an
important portion of new species established by professional or non-professional (amateur) taxonomists is based on specimens
preserved in the collections of museums, universities and other scientific institutions (Winston, 1999; Fontaine et al., 2012). Such collections also include many species that are still undescribed (Blackman & Eastop, 2006).
Specimens of two samples in the aphid collection of the Natural History Museum in London (BMNH) belonging to genus Uroleucon Mordvilko, 1914 (Hem. Aphididae Aphidinae Macrosiphini) have been studied, one from Lebanon and another from Iran. The slides
of both samples had been labelled as new species by D. Hille Ris Lambers, and one of them is mentioned by Blackman & Eastop
(2006, page 229).
Uroleucon currently includes 235 valid species and several subspecies (Favret, 2013; Nieto Nafría & Pérez Hidalgo, 2013), being one the largest genera of the tribe Macrosiphini.
Material and methodsTOP
The studied samples do not have a distinctive number; one is from Lebanon and the other from Iran.
Lebanese sample: “Carthamus flavescens, Arec, 26-XI-1971, Talhouk leg. (nr. 39)”; 14 apterous viviparous females, 4 alate viviparous females and 6 immature specimens. This sample is mentioned
by Blackman & Eastop (2006 [2013], page 229) as Uroleucon sp. near jaceae. The locality of Arec is in the Bekaa valley.
Iranian sample: “Helichrysum sp., Demavend Village, 12-VI-1960, van den Bosch leg. (IR 132)”; 25 apterous viviparous females, 5 alate viviparous females and 22 immature specimens. This sample is not mentioned
by Blackman and Eastop (op. cit.). We think that the locality “Demavend” is a misspelling for Damavand, a locality near Mount Damavand.
Measurements of the slide-mounted specimens were made according to Nieto Nafría & Mier Durante (1998) with an ocular micrometer. The measurements are lengths except when indicated that they are a width or diameter.
The photomicrographs were taken with a Leica DC digital camera with IM 1000 version 1.10 software.
Results and DiscussionTOP
The Lebanese specimens above mentioned have been identified as Uroleucon jaceae (Linnaeus, 1758). We do not put a subspecific name on these specimens, because the species is very variable and the subspecies
are badly defined.
The specimens of the Iranian sample are different to the Lebanese specimens, mainly in the shape and ornamentation of the
siphunculi. We consider that these aphids belong to a new species of the genus Uroleucon, subgenus Uromelan.
Uroleucon helichrysi sp. n.
urn:lsid:zoobank.org:act:20A34165-03B9-425A-8B8E-F4E7A8DCEF5D
TYPES. Holotype: Apterous viviparous female, number 3 of the measurement series, IRAN, Mazanderan province, Damavand, 12-VI-1960,
on Helichrysum sp., v.d.Bosch leg. (IR 132). Paratypes: 24 apterous viviparous females and 5 alate viviparous females, same data. Collection of the Natural
History Museum (BMNH), London, United Kingdom.
APTEROUS VIVIPAROUS FEMALES (Table 1, Fig. 1). From 39 apterous viviparous females. Metric and meristic features in Table 1. Colour in life unknown. When mounted mostly pale yellowish brown with striae on dorsum of thorax and abdomen. Marginal tubercles
usually present in prothorax and abdominal segments 2-4; they are small, similar in size to neighbouring seta sockets. Setae
thick and pale, most of them with blunt or evanescent apex. Head brown, and dorsally with very faint striae. Frontal sinus very deep and fronto-medial tubercle small or inconspicuous (Fig. 1A). Antennae brown to dark brown, usually with the proximal portion of segment III paler; proximal portion of segment III
and most of IV delicately ornamented, segments V and VI imbricated. Secondary sensoria scattered on proximal 50-66% of segment
III; they are circular, small and somewhat protuberant. Rostrum reaching hind coxae, dark brown; ultimate rostral segment
triangular; proximal ones with spinules. Thorax with striate pale brown marginal sclerites in addition to the brown spinal and pleural setiferous scleroites. Coxae, distal
1/3-1/2 of femora, very proximal portion and distal 2/5-1/3 of tibiae, and tarsi dark brown. Abdomen with dorsal setiferous scleroites (Fig. 1B), with neighbouring scleroites on abdominal segment 7 and 8 sometimes coalescent; postsiphuncular and antesiphuncular (sometimes
broken) patches; and spiracular sclerites small and usually paler than the setiferous scleroites; the intersegmental sclerites
are inconspicuous. Siphunculi dark brown (Fig. 1C), cylindrical with enlarged base and curved outward, and without both preapical incision and flange; cells of the reticulate
part very small (Fig. 1E); part proximal to reticulation densely covered with groups of spinules or scales. Genital plate light brown with brown
spots. Anal plate similar in colour to cauda, which is lanceolate and as dark as siphunculi (Fig. 1D).
ALATE VIVIPAROUS FEMALES (Table 1, Fig. 2). From 9 alate viviparous females. Metric and meristic features in Table 1. Colour in life unknown; when mounted similar to apterae (Figs. 2A, 2B). Antennae brown to dark brown, usually with the proximal portion of segment III paler (Fig. 2C). Secondary sensoria (60-82) circular small and somewhat protuberant (Figs. 2C, 2D). Abdomen with dorsal setiferous sclerites (Fig. 2B). Siphunculi dark brown (Fig. 2B), similar to those of the apterae, without both preapical incision and flange. Cauda is lanceolate and as dark as siphunculi.
BIONOMICS. The only known host plant of the aphid is an unidentified species of Helichrysum (Asteraceae); the capacity of this aphid to colonize other composites is unknown.
DISTRIBUTION. The new species is only known from one Iranian locality, in Mazanderan province.
ETYMOLOGY. The specific name helichrysi is the genitive of the name of the host plant of the new aphid species.
TAXONOMIC DISCUSSION. The assignment of the species to genus Uroleucon and subgenus Uromelan Mordvilko, 1914 is unquestionable, given the characteristics described above.
|
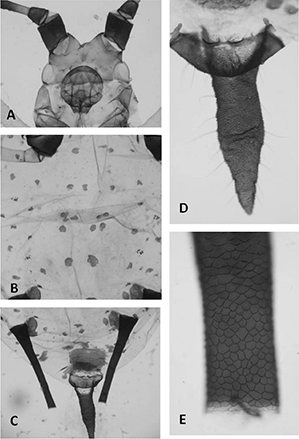 Fig. 1.— Apterous viviparous females: Uroleucon (Uromelan) helichrysi sp. n.: head (A), anterior abdominal segments (B), posterior abdominal segments (C), cauda (D), and apical part of siphunculi (F). Fig. 1.— Apterous viviparous females: Uroleucon (Uromelan) helichrysi sp. n.: head (A), anterior abdominal segments (B), posterior abdominal segments (C), cauda (D), and apical part of siphunculi (F).
Fig.
1.— Hembras vivíparas ápteras: Uroleucon (Uromelan) helichrysi sp. n.: cabeza (A), segmentos anteriores del abdomen (B), parte posterior del abdomen (C), cola (D) y parte apical del cornículo
(F).
|
|
Table 1.– Metric and meristic features of apterous and alate viviparous females of Uroleucon helichrysi sp. n.
Tabla 1.– Características métricas y merísticas de las hembras vivíparas ápteras y aladas de Uroleucon helichrysi n. sp.
|
apterous viviparae |
alate viviparae |
| Body (cauda included) (mm) |
3.775-4.625 |
3.550-4.500 |
| Antenna (mm) |
3.810-3.990 |
3.410-4.520 |
| Antennal segment III [Ant.III] (mm) |
0.98-1.08 |
0.88-1.12 |
| Antennal segment IV (mm) |
0.72-0.85 |
0.64-0.92 |
| Antennal segment V (mm) |
0.60-0.72 |
0.56-0.80 |
| Antennal segment VI, base [Ant.VI base] (mm) |
0.16-0.19 |
0.16-0.21 |
| Antennal segment VI, terminal processus [Ant.VI.t.p.] (mm) |
0.85-1.03 |
0.90-1.23 |
| Ultimate rostral segment [U.r.s.] (mm) |
0.19-0.21 |
0.19-0.21 |
| Hind Femur [H.F.] (mm) |
1.40-1.50 |
1.08-1.48 |
| Hind Tibia [H.T.] (mm) |
2.43-2.55 |
2.03-2.70 |
| Second segment of hind tarsi [H.t.2] (mm) |
0.15-0.17 |
0.13-0.16 |
| Siphunculus [SIPH.] (mm) |
1.00-1.13 |
0.82-1.18 |
| Cauda (mm) |
0.60-0.70 |
0.46-0.58 |
| Body / H.F. (times) |
2.60-3.25 |
2.91-3.30 |
| Body / H.T. (times) |
1.55-1.89 |
1.60-1.75 |
| Antenna / Body (times) |
0.87-1.00 |
0.96-1.11 |
| Ant.III / Ant.VI base |
5.44-6.31 |
5.33-5.94 |
| Ant.III / U.r.s. |
4.67-5.35 |
4.51-5.63 |
| Ant.VI.t.p. / Ant.III (times) |
0.81-1.01 |
1.02-1.12 |
| Ant.VI.t.p. / Ant.VI base (times) |
4.58-6.00 |
5.63-6.15 |
| U.r.s. / basal width of U.r.s. (times) |
2.67-3.15 |
2.79 |
| U.r.s. / Antennal segment I (times) |
0.87-1.05 |
0.95-1.08 |
| U.r.s. / Ant.VI base (times) |
1.08-1.31 |
0.98-1.22 |
| U.r.s. / interocular dorsal distance (times) |
0.43-0.53 |
0.46-0.49 |
| U.r.s. / H.t.2 (times) |
1.15-1.26 |
1.31-1.50 |
| SIPH. / Body (times) |
0.22-0.28 |
0.23-0.28 |
| SIPH. / Ant.III (times) |
0.93-1.12 |
0.93-1.17 |
| SIPH. / interocular dorsal distance (times) |
2.15-2.68 |
2.20-2.61 |
| SIPH. / basal width of SIPH. (times) |
3.89-4.74 |
4.56-5.62 |
| SIPH. / minimal width of SIPH. (times) |
7.14-9.64 |
6.83-12.42 |
| Basal / minimal widths of SIPH. (times) |
1.64-2.18 |
1.50-2.35 |
| Basal width of SIPH. / diameter of Hind tibia at its middle (times) |
1.22-2.00 |
1.46-2.00 |
| Reticulated portion of SIPH. / SIPH. (times) |
0.18-0.27 |
0.21-0.25 |
| Cauda / SIPH. (times) |
0.58-0.69 |
0.47-0.56 |
| Cauda / basal width of Cauda (times) |
2.36-2.65 |
1.96-2.33 |
| Secondary sensoria on Ant.III (number) |
31-52 |
60-82 |
| Setae on U.r.s. (number) |
7-10 |
6-8 |
| Setae on abdominal segment 3rd, dorsum (number)
|
18-27 |
23-34 |
| Setae on abdominal segment 7th, dorsum (number)
|
6-11 |
8-12 |
| Setae on abdominal segment 8th (number)
|
4-6 |
4-6 |
| Setae on cauda (number) |
20-28 |
21-25 |
| Setae on subgenital plate, discal (number) |
4-8 |
5-12 |
| Setae on subgenital plate, marginal (number) |
18-23 |
16-18 |
| Rows on reticulate portion of siphunculi |
20-25 |
11-18 |
| Longest seta on …
|
|
|
| … Antennal segment III (μm) |
38-50 |
28-45 |
| … Antennal segment III / basal width of Ant.III (times) |
0.6-0.8 |
0.5-0.9 |
| … Vertex (μm) |
55-75 |
50-60 |
| … Vertex / basal width of Ant.III (times) |
0.8-1.2 |
0.9-1.1 |
| … Hind trochanter, posterior (μm) |
50-75 |
45-50 |
| … Hind trochanter, posterior / (/) tr. (times) |
0.5-0.7 |
0.5-0.6 |
| … H.F., dorsum (μm) |
40-55 |
30-40 |
| … H.F., venter (μm) |
45-100 |
40-60 |
| … H.F., dorsum / basal width of Ant.III (times) |
0.6-0.8 |
0.6-07 |
| … H.F., venter / basal width of Ant.III (times) |
0.8-1.5 |
0.7-1.2 |
| … H.T., dorsum at middle (μm) |
50-60 |
35-45 |
| … H.T., dorsum / diameter of H.T at middle |
0.5-0.8 |
0.6-0.7 |
| … abdominal segment 3rd, spinal or pleural (μm)
|
38-80 |
48-55 |
| … abdominal segment 3rd / basal width of Ant.III (times)
|
0.6-1.3 |
0.8-1.1 |
| … abdominal segment 8th (μm)
|
60-83 |
60-80 |
| … abdominal segment 8th / basal width of Ant.III (times)
|
0.9-1.3 |
1.1-1.6 |
|
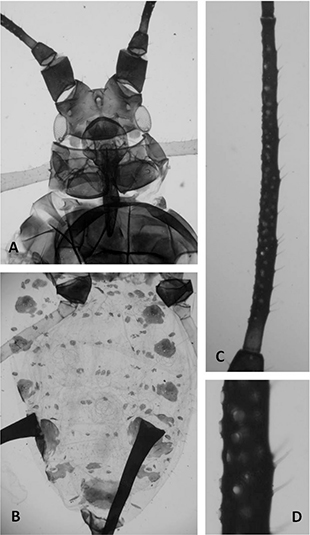 Fig. 2. — Alate viviparous females: Uroleucon (Uromelan) helichrysi sp. n.: head and thorax (A), abdominal segments (B), antennal segment III (C), and detail of secondary sensorial of antennal segment
III (D). Fig. 2. — Alate viviparous females: Uroleucon (Uromelan) helichrysi sp. n.: head and thorax (A), abdominal segments (B), antennal segment III (C), and detail of secondary sensorial of antennal segment
III (D).
Fig. 2.— Hembras vivíparas aladas: Uroleucon (Uromelan) helichrysi sp. n.: cabeza y parte del tórax (A), abdomen (B), antenómero III (C) y detalle de los sensorios secundarios del antenómero III
(D).
|
|
The principal characters involved in the comparison of U. helichrysi sp. n. (this work) to other Palaearctic species of Uromelan (original descriptions) are the tarsal formula (5.5.5), the number of caudal setae (20-28), the size of the siphuncular cells
and the presence of marginal tubercles on abdominal segments 2-4.
Most Palaearctic Uromelan species have 5 setae on the first tarsal segments, but only five of them have 20 or more caudal setae (Hille Ris Lambers,
1939; Miyazaki, 1971; Takahashi, 1962; Rezwani & Lampel, 1987; Heie, 1995; Lee et al., 2002): U. aeneum (Hille Ris Lambers, 1939), U. caspicum Rezwani & Lampel, 1987; U. cephalonopli (Takahashi, 1962), U. giganteum (Matsumura, 1918) and U. jaceae (Linnaeus, 1758).
U. caspicum and U. giganteum (Matsumura, 1918) have many more setae on the cauda than U. helichrysi (24-41 and 40-60 respectively). U. cephalonopli has, in comparison with U. helichrysi, a long and narrow ultimate rostral segment (0.22-0.30 mm and 1.4-1.6 times second segment of hind tarsus), and the siphunculi
and cauda are different in shape (see Lee et al., 2002, fig. 227). U. jaceae lacks abdominal marginal tubercles, and the cells of the siphuncular reticulation are larger than those in U. helichrysi, as in U. aeneum. The metric and meristic characteristics of U. aeneum are very similar to those of the new species, but the shape and reticulation of the siphunculi are very different. In addition
none of these species live on Helichrysum.
The “Key to apterae on Helichrysum” by Blackman & Eastop (2006, p. 510) can be modified to include U. helichrysi sp. n. as follows, respecting terminology, abbreviations and expressions.
| 15. |
[without modifications]........ Uroleucon cichorii |
| - |
SIPH 1.5-2.1× cauda, which is dark Antesiphuncular sclerites absent .................15B |
| 16B. |
SIPH 1.8-2.1× cauda, which has 11-20 hairs ......................Uroleucon compositae (or gobonis) |
| - |
SIPH 1.5-1.7× cauda, which has 20-28 hairs................................................... Uroleucon helichrysi |
AcknowledgmentsTOP
The authors wish to thank the Natural History Museum of London for the facilities to study the slides of its collection.
ReferencesTOP
| ○ |
Blackman, R. L. & Eastop, V. F., 2006. Aphids on the World’s herbaceous plants and shrubs. Volume 1 Host Lists. Volume 2 The Aphids. John Wiley & Sons, Ltd. Chicheter (U. K.). 8+1024 pp. Actualized 2013: Aphids on the World’s plants. An online identification and informative guide. Web service available online at http://www.aphidsonworldsplants.info [consulted April 2013].
|
| ○ |
Favret, C., 2013. Aphid Species File. Version 1.0/4.1. [Consulted April 2013]. <http://Aphid.SpeciesFile.org>.
|
| ○ |
Fontaine, B., van Achterberg, K., Alonso-Zarazaga, M. A. Araujo, R., Asche, M., Aspöck, H., Aspöck, U., Audisio, P., Aukema,
B., Bailly, N., Balsamo, M., Bank, R. A., Belfiore, C., Bogdanowicz, W., Boxshall, G., Burckhardt, D., Chylarecki, P., Deharveng,
L., Dubois, A., Enghoff, H., Fochetti, R., Fontaine, C., Gargominy, O., Gómez López, M. S., Goujet, D., Harvey, M. S., Heller,
K. G., van Helsdingen, P., Hoch, H., De Jong, Y., Karsholt, O., Los, W., Magowski, W., Massard, J. A., McInnes, S. J., Mendes,
L. F., Mey, E., Michelsen, V., Minelli, A., Nieto Nafría, J. M., van Nieukerken, E. J., Pape, T., De Prins, W., Ramos, M.,
Ricci, C., Roselaar, C., Rota, E., Segers, H., Timm, T., van Tol, J., Bouchet, P., 2012. New Species in the Old World: Europe
as a Frontier in Biodiversity Exploration, a Test Bed for 21st Century Taxonomy. PLoS ONE, 7(5): e36881, 7 pp. http://dx.doi.org/doi:10.1371/journal.pone.0036881.
|
| ○ |
Heie, O. E., 1995. The Aphidoidea (Hemiptera) of Fennoscandia and Denmark. VI. Family Aphididae: Part 3 of tribe Macrosiphini
of subfamily Aphidinae, and family Lachnidae. Fauna Entomologica Scandinavica, 31: 1-217.
|
| ○ |
Hille Ris Lambers, D., 1939. Contributions to a monograph of the Aphididae of Europe. II. The genera Dactynotus Rafinesque, 1818; Staticobium Mordvilko, 1914; Macrosiphum Passerini, 1860; Masonaphis nov. gen.; Pharalis Leach, 1826. Temminckia, 4: 1-134 & 6 plates.
|
| ○ |
Lee, S. H., Holman, J. & Havelka, J., 2002. Illustrated catalogue of Aphididae in the Korean Peninsula. Part I, Subfamily Aphidinae (Hemiptera Sternorryncha). In: K.-T. Park (ed.). Insects of Korea [9]. Korea Research Institute of Bioscience & Biotechnology and the Center for Insects Systematics. [Without locality]. 331
pp.
|
| ○ |
Miyazaki, M., 1971. A revision of the tribe Macrosiphini of Japan (Homoptera: Aphididae, Aphidinae). Insecta Matsumurana, 34(1): 1-247.
|
| ○ |
Nieto Nafría, J. M. & Mier Durante, M. P., 1998. Hemiptera Aphididae I. In: M. A. Ramos et al. (eds.). Fauna Ibérica, volumen 11. Museo Nacional de Ciencias Naturales (CSIC). Madrid. 424 pp.
|
| ○ |
Nieto Nafría, J. M. & Pérez Hidalgo, N., 2013. Two new Palaearctic species of Uroleucon (Hemiptera: Aphididae) from the collection of the Muséum national d’Histoire naturelle de Paris. Boletín de la Asociación Española de Entomología, 37(1-2): 31-47.
|
| ○ |
Rezwani, A. & Lampel, G., 1987. Two new species of Uroleucon Mordv. (Homoptera: Aphididae) from Iran. Mitteilungen der Schweizerischen Entomologischen Gesellschaft, 60: 325-330.
|
| ○ |
Takahashi, R., 1962. Key to Japanese species of Dactynotus, with descriptions of four new species (Aphididae, Homoptera). Kontyû, 30(2): 73-81.
|
| ○ |
Winston, J. E., 1999. Describing Species, Practical Taxonomic Procedure for Biologists. Columbia University Press. New York. 541 pp.
|
Fig. 1.— Apterous viviparous females: Uroleucon (Uromelan) helichrysi sp. n.: head (A), anterior abdominal segments (B), posterior abdominal segments (C), cauda (D), and apical part of siphunculi (F).